2012
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Íîâîñòè |
| Ïåðâûé öèðêóëÿð |
| Âòîðîé öèðêóëÿð |
| Ðåãèñòðàöèÿ |
| Îôîðìëåíèå òåçèñîâ |
| Òåçèñû |
| Ïðîãðàììà |
| Ó÷àñòíèêè |
| Ðàçìåùåíèå |
| Ýêñêóðñèè |
| Ïðîåçä |
| Âàæíûå äàòû |
| Îðãêîìèòåò |
| Îáðàòíàÿ ñâÿçü |
|
Òåçèñû ìåæäóíàðîäíîé êîíôåðåíöèè |
Abstracts of International conference |
||||||||||||||||||||||||||||||||||||||||||||
|
Mineralogy of metacarbonate xenolith from alkali basalt, E.Eifel, Germany Sharygin V.V. V.S.Sobolev Institute of Geology and Mineralogy SD RAS, Novosibirsk, Russia sharygin@igm.nsc.ru
Metacarbonate xenoliths in volcanic rocks are natural analogs of cement clinkers and carbonate rocks, transformed by combustion metamorphism in natural and technogenic conditions (Sokol et al., 2005). The Bellerberg volcano, E.Eifel, Germany, is one of impressive localities of such xenoliths. These metacarbonate xenoliths very strongly vary in mineral composition and sometimes contain abundant retrograde phases. This report is devoted to one of fresh samples (E-2011) with unique mineralogy (Table 1). This specimen contains two potentially new mineral species: “Fe-shulamitite”, Fe- analog of shulamitite (Sharygin et al., 2011), and K-Ba-sulfide intermediate member between djerfisherite K6(Fe,Cu,Ni)25S26Cl and owensite (Ba,Pb)6(Cu,Fe,Ni)25S27 (Laflamme et al., 1995). Late secondary phases are not abundant and confined to vugs and healed fissures in the xenolith (ettringite, hydrated Ca-silicates, calcite).
Table 1. Primary minerals found in the metacarbonate xenolith, sample E-2011, E. Eifel.
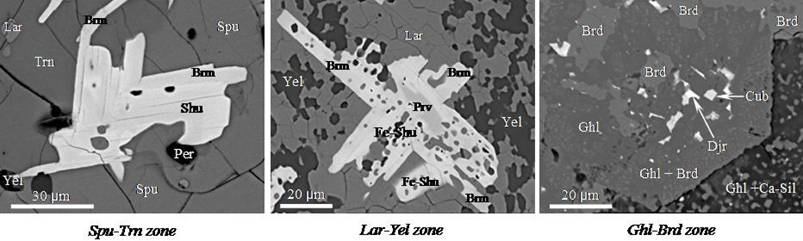
The xenolith has pronounced zonality near the contact with alkali basalt, and the contact is well shown due to brown zone (Fig. 1) mainly represented by minerals of the melilite group (from alumoakermanite to gehlenite). Reddish-brown tint of other xenolith’s part is owing to abundant oxide ore phases (Ca-ferrites, perovskite, spinel group minerals). Other zones are visually indistinguishable and fixed only by the change of mineral assemblages. Three additional zones may be divided clearly in the predominance of the principal minerals: gehlenite-bredigitic, larnite-ye’elimitic and spurrite-ternesitic (Fig. 1-2). The first zone contains dominant Al- and Mg-bearing silicates (gehlenite, bredigite) and minor Cu-Fe-sulfides (cubanite, K-Fe-sulfide), barite and Mg-Fe-spinels. The second zone is abundant in ordinary Ca-silicate (larnite), Ca-sulfate-aluminate (ye’elimite), periclase and rare chalcocite. The third zone is represented by Ca-silicates with additional [SO4] è [CO3] groups (ternesite, spurrite), whereas the amount of ye’elimite, periclase and larnite is decreased and sulfides are absent.
Fig. 1. Zonation in metacarbonate xenolith, sample E-2011, E. Eifel. Mineral are listed in their abundance in zones.
The total amount of oxide ore phases in these zones is approximately equal, but their ratio is changed in the associations as far as the contact with basalt (Fig. 2): perovskite + magnesioferrite, perovskite + “Fe-shulamitite”, brownmillerite + srebrodolskite, perovskite + shulamitite + “Fe-shulamitite” + brownmillerite ± srebrodolskite ± magnesioferrite, shulamitite + brownmillerite ± perovskite. In the case of the coexistence of one series minerals (shulamitite - “Fe-shulamitite”, brownmillerite - srebrodolskite), the Al-richer phase is crystallized in first order. In spurrite-ternesitic zone, where the abundance of ye’elimite is minimal, Al-rich Ca-ferrites (shulamitite, brownmillerite) is more stable, and perovskite occurs rarely as relic in shulamitite.
Fig. 2. Mineral composition in the individual zones of the xenolith (scanning microscopy). Symbols: Trn - ternesite, Spu - spurrite, Lar - larnite, Per - periclase, Yel - ye’elimite, Brm - brownmillerite, Shu - shulamitite, Fe-Shu - “Fe-shulamitite”, Prv - perovskite, Ghl - gehlenite, Brd - bredigite, Ca-Sil - hydrated Ca-silicates, Djr - “Ba-djerfisherite”, Cub - cubanite.
Some chemical features for minerals may be indicated towards to the contact with basalt. The SrO concentration is increased in all Ca-containing minerals (up to 2-4 wt.%). Periclase is getting richer in FeO up to the appearance of the solid phase decay structures (wüstite or magnesioferrite). The content of Fe2O3 is increased in Ca-ferrites. Ba- and Cu-containing phases are common only of the zones nearby the contact. Thus, pronounced metasomatic zonation in mineral chemistry and mineral assemblages is revealed in the studied Eifel xenolith. It should be noted that similar localization of minerals enriched in Ba, Sr and other trace elements to the contact zones with silicate melt was previously described for metacarbonate rocks from Chegem and Donetsk (Sharygin, 2011; Galuskin et al., 2008; 2011). In petrologic aspect the study of metacarbonate xenoliths in volcanic rocks always emerges a question: could silicate melt totally assimilate them or reactionary relations and solid phase transformation are available only? In the case of Eifel the protolith was represented by limestone, which fragments were trapped by alkaline magma on shallow depths during ascending. The melting of such high-Ca protolith is possible only at temperature higher than 1350-1400oC. Assuming olivine-free association of the host basalt (close to nephelinite) the liquidus temperature of alkaline melt was approximately 1300îÑ. Moreover, temperature gradient should be taken into account during transformation of carbonate xenolith. The absence of reliable indicator minerals in the xenolith allows only the rough estimations in the broad temperature range (900-1200îÑ), what corresponds to HT region of the spurrite-merwinite facies (Sokol et al., 2005). The association perovskite + shulamitite or “Fe-shulamitite (Sharygin et al., 2008) can not be used for temperature estimations of individual mineral assemblages in the studied xenolith because it is not in equilibrium. The relationships of phases indicate that shulamitite and “Fe-shulamitite” are later phases than perovskite, and they grow on perovskite and even replace it (Fig. 2). The superior temperature limit is based on that in high-Ca systems with SiO2 Ca-ferrites and alumoferrites of different composition (Ca2(Fe,Al)2O5, CaFe2O4, etc.) are stable only up to 1200-1210îÑ. The increasing of temperature provokes them to react with Ca-silicates with formation of Ca-silicoferrites of the aenigmatite-dorrite group (Scarlett et al., 2004). These specific minerals are not found in the xenolith. The author would like to thank B.Ternes and W.Schüller (Germany) for donating of metacarbonate xenoliths from E. Eifel for detailed studies.
References Galuskin E.V., Galuskina I.O., Gazeev V.M., Dzierżanowski P., Prusik K., Pertsev N.N., Zadov A.E., Bailau R., Gurbanov A.G. Megawite, CaSnO3: a new perovskite-group mineral from skarns of the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia // Mineralogical Magazine. 2011. V. 75. P. 2563–2572. Galuskin E.V., Gazeev V.M., Armbruster T., Zadov A.E., Galuskina I.O., Pertsev N.N., Dzierzanovski P., Kadiyski M., Gurbanov A.G., Wrzalik R., Winiarski A. Lakargiite CaZrO3: A new mineral of the perovskite group from the Northern Caucasus, Kabardino-Balkaria, Russia // American Mineralogist. 2008. V. 93. P. 1903-1910. Laflamme J.H.G., Roberts A.C., Criddle A.J., Cabri L.J. Owensite, (Ba,Pb)6(Cu,Fe,Ni)25S27, a new mineral species from the Wellgreen Cu-Ni-Pt deposit, Yukon // The Canadian Mineralogist. 1995.V. 33. P. 665-670. Scarlett N.V.Y., Pownceby M.I., Madsen I.C., Christensen A.N. Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter // Metallurgical and Materials Transactions B. 2004. V. 35B. P. 929-936. Sharygin V.V. Lakargiite and minerals of the perovskite-brownmillerite series in metacarbonate rocks from Donetsk burned dumps // Proceedings of Donetsk National Technical University, Mining and Geological Series. V. 15 (192). P. 113-123 (in Russian). Sharygin V.V., Lazic B., Armbruster T., Murashko M.N., Wirth R., Galuskina I.O., Galuskin E.V., Vapnik, Y. Shulamitite, IMA 2011-016. CNMNC Newsletter No. 10. October 2011. P. 2552 // Mineralogical Magazine. 2011. V. 75, No. 5. P. 2549-2561. Sharygin V.V., Sokol E.V., Vapnik Ye. Minerals of the pseudobinary perovskite-brownmillerite series from combustion metamorphic larnite rocks of the Hatrurim Formation (Israel). Russian Geology and Geophysics. 2008. V.49 (10). P. 709-726. Sokol E.V., Maksimova N.V., Nigmatulina E.H., Sharygin V.V., Kalugin V.M. Combustion metamorphism. Novosibirsk: PH SD RAS. 2005. 284 p. (in Russian). |
|||||||||||||||||||||||||||||||||||||||||||||