2012
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Новости |
| Первый циркуляр |
| Второй циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
|
Тезисы международной конференции |
Abstracts of International conference |
|
Some geochemical features of carbonatite baddeleyite from Kovdor Massif (Kola Peninsula) Rodionov N.V.*, Belyatsky B.V **, Antonov A.V.*, Simakin S.G.***, Kapitonov I.N.*, Sergeev S.A.* *CIR VSEGEI, S.Petersburg, Russia, **VNIIOkeangeologia, S.Petersburg, Russia, ***YaB PhThIAN, Yaroslavl, Russia bbelyatsky@mail.ru
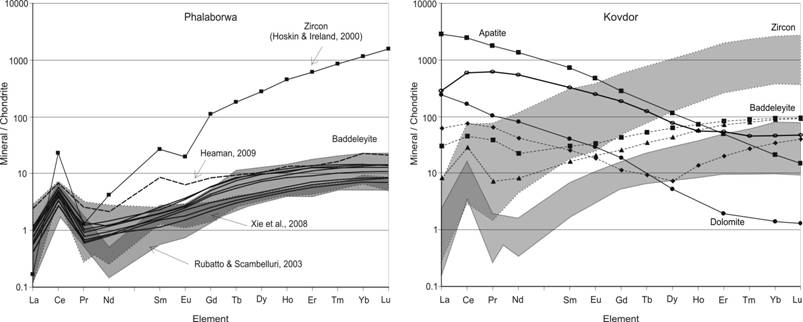
Recently baddeleyite (ZrO2) has been introduced into practice of U-Pb dating of basic, ultrabasic and alkaline intrusive rocks [Heaman, 2009; Rodionov et al., 2012]. It is known that its crystallization from magmatic melts is determined by low SiO2 activity at rather high Zr concentrations and at the same time its occurrence together with zircon in polyphase alkaline-ultrabasic intrusions evidence to more complicated and variable genesis of this mineral. In many cases composition of trace elements may be used as genetic indicator for the origin not only of rock-forming minerals but accessory minerals as well. Zircon trace elements composition is widely used in geochemistry [Hoskin, Ireland, 2000], but as for baddeleyite, its geochemical characteristics which reflect the peculiarities of crystallization and origin are still insufficiently known [Klemme, Meyer, 2003]. For example, the published data on composition of rare-earth elements (obtained both by in-situ and bulk sample analysis of this mineral) are debatable and in many cases contradictory. Results of in-situ analysis of separate baddeleyite grains prove the main geochemical properties of this mineral, but even for baddeleyite from Phalaborwa massif which is often used as geochemical standard, the results from different laboratories vary significantly [Rodionov et al., 2012]. In particular, the shape and value of Ce anomaly differs more than in order, in many REE patterns the Eu anomaly is absent and the range of REE fractionation differs from the results obtained by other analytical methods. At the same time, many scientists consider baddeleyite from the Kovdor massif as a potential standard for geochronological studies. This has stimulated the present study of in-situ geochemical composition of representative baddeleyite grains from the Kovdor massif. It is well known [Rimskaya-Korsakova, Dinaburg, 1964; Kirnarsky, 1979; Kopylova et al., 1980] that baddeleyite in the rocks of alkaline-ultrabasic Kovdor complex is ubiquitous mineral and it is formed at all stages of rocks formation from olivinites to ore phoscorites and postmagmatic syenites [Dudkin, Kirnarsky, 1994; Krasnova, Kopylova, 1988]. Morphology of baddeleyite varies greatly – from large, several centimeters, black crystals to light colored yellow semitransparent sub-millimeter grains [Kirnarsky, 1979]. We have studied in-situ compositions of more than 50 different in morphology baddeleyite grains from the carbonatite and phoscorite samples from the Kovdor massif and 20 baddeleyite grains from the Phalaborwa carbonatite massif (South Africa) for comparison and test of the approach applicability (ion microprobe CAMECA IMS-4F and laser-ablation with ISP-MS Element2). Our results of baddeleyite REE composition are presented in the summary diagrams on Fig.1 and the most important are formulated below.
Fig.1. REE patterns for baddeleyite from carbonatite complexes: (а) Phalaborwa (S.Africa), for comparison data from Heaman, 2009; Hoskin, Ireland, 2000; Rubatto, Scambelluri, 2003; Xie et al., 2008 are shown; and (b) Kovdor (Kola Peninsula), open circles – ISP-MS data for 100 mg baddeleyite sample.
As above mentioned, the published data on baddeleyite trace element composition are scarce even for the “Phalaborwa” U-Pb standard [Heaman, 2009; Rodionov et al., 2012]. According to a few studies on the “Phalaborwa” standard (∑REE = 10.6–22.4 ppm) baddeleyite REE composition displays significantly lower HREE than those typical for magmatic zircon and other baddeleyites and less fractionated: (Lu/La)n = 8.4–20.1, (Lu/Gd)n= 2.5–4.2, (Sm/La)n= 2.4–3.5 [Reischmann et al., 1995; Rubatto, Scambelluri, 2003; Xie et al., 2008] (Fig. 1a). This agrees well with experimental data on the composition of baddeleyite crystallized from carbonatitic melt [Klemme, Meyer, 2003]. Our trace element data for the Phalaborwa baddeleyite are quite similar to the earlier published in-situ analyses and reflect relatively limited variation of their compositions and acceptable reproducibility of the chosen microanalysis methods (Fig. 1a). But the LREE patterns for Phalaborwa baddeleyite are considerably different from the data of Reischmann et al. (1995), obtained with a combination of spark source and thermal ionization mass spectrometry techniques, with an unusual U-shaped REE pattern without a Eu-anomaly. It is supposed that analyses are compromised by inclusions and impurities in the studied baddeleyite separates [Reischmann et al., 1995]. But the chondrite-normalized REE patterns for studied Kovdor baddeleyite are more diverse with some of them resemble the published U-shaped distribution for Phalaborwa baddeleyite (Fig.1b). In accordance with abovementioned suggestions we attribute these deviated REE distributions to the influence of the micro-inclusions (mostly apatite and carbonates), the presence of which was confirmed by SEM-EDX analyses for some baddeleyite grains. REE patterns for large apatite and dolomite inclusions located inside baddeleyite crystals confirm such interpretation, since their REE composition patterns exhibit strong LREE enrichment, similar to those reported earlier by Kempe and Götze (2002) and Arzamastsev et al. (2009) for these minerals from the Kovdor and Phalaborwa massifs [Landa et al., 1983; Dawson, Hinton, 2003]. Moreover, simple mass-balance calculations show that no more than 5-10 wt.% of apatite/dolomite addition to baddeleyite composition would result in observed REE distribution (dashed lines on Fig.1b). One more argument in favor of such interpretation of the data are the results of analysis of bulk baddeleyite sample (100mg, ISP-MS from the solution) represented on the same diagram (Fig.1b, open circles). At the same time trace element distributions for the studied carbonatite and foscorite-hosted baddeleyite do not demonstrate any significant HREE enrichment ((Lu/La)n = 23.8, (Lu/Gd)n= 5.4) that is typical for baddeleyite of definite metamorphic origin [Rubatto, Scambelluri, 2003]. But ∑REE varies considerably – from 7.8 to 160 ppm for Kovdor baddeleyite and [Nb] from 980 to 2660, [Y] from 19.6 to 41.1, [Ti] from 17.6 to 47.9, [Hf] from 7440 to 12160, [Th] from 1.96 to 13.7, [U] from 121 to 774 ppm, but [Sr] from 1.34 to 1.89 and [Ba] from 0.23 to 0.36 ppm which is quite unlike to those for Phalaborwa baddeleyite. Moreover, there is surprising difference in the shape of a Ce positive anomaly for baddeleyite and zircon REE patterns (Fig. 1). Zircon LREE normalized distribution generally demonstrates the existence of a La-Ce-Pr peak. The shape of this peak is determined by relative value of chondrite-normalized La, Ce, Pr and Nd: Lan£Prn<Ndn<<Cen. By comparison, baddeleyite REE patterns are characterized by La-Ce-Nd anomaly also that is determined by relatively reduced Nd content (Lan£Ndn<Prn<<Cen). But in-situ baddeleyite composition data are controversial even for Phalaborwa baddeleyite standards. Rubatto and Scambelluri (2003) reported the first type of Ce positive anomaly (zircon type); while Xie et al. (2008) obtained both LREE distributions for Phalaborwa baddeleyites. Trace element compositions for baddeleyite from other than carbonatite rocks show analogous complex behavior [Rubatto, Scambelluri, 2003]. We produced Phalaborwa baddeleyite REE patterns that have only zircon-type positive Ce anomaly (Fig. 1a) but Kovdor baddeleyite data confirm also the presence of both La-Ce-Pr and La-Ce-Nd type of Ce positive anomaly (Fig. 1b). The significance and reason for such Nd behavior in baddeleyite crystal structure is not clear because experimental data is not sufficient for this [Klemme, Mayer, 2003] but a possible explanation could be connected with the magma’s middle REE exhaustion during crystallization of such REE-rich minerals as F-apatite, REE-pyrochlore, calzirtite, zirconolite and others. To prove this hypothesis it is necessary to carry out further investigations of, in particular, the character of baddeleyite composition evolution accompanying the development in time of magmatic and ore-formation processes. The present study of geochemical features of individual baddeleyite crystals from phoscorites and carbonatites of the Kovdor massif (Fig.1) has demonstrated the excess heterogeneity of their composition in comparison to those variation for Phalaborwa massif (South Africa) baddeleyite which is determined not only by the presence of microinclusions of carbonates and apatite in the samples studied but, perhaps, by the difference in the grains studied origin. But this could not exclude the possibility that baddeleyite crystals formed within the rocks and ores of one magmatic stage and representing only one morphological type are characterized by narrow composition variations and have the single concordant U-Th-Pb isotope age, i.e. they posses all the necessary features for isotope-geochemical and geochronological standard.
References: Arzamastsev A.A., Arzamastseva L.V., Bea F., Montero P. Trace elements in minerals as indicators of the evolution of alkaline ultrabasic Dike Series: LA-ICP-MS Data for the magmatic provinces of Northeastern Fennoscandia and Germany. Petrology. 2009. V.17(1). P. 46–72. Dudkin O.B., Kirnarsky Yu.M. Kovdor Massif Deposits. Geology of Ore Deposits. 1994. V.36. №1. P. 31-41. Dawson J.B., Hinton R.W. Trace-element content and partitioning in calcite, dolomite and apatite in carbonatite, Phalaborwa, South Africa. Mineralogical Magazine. 2003. V.67(5). P. 921–930. Heaman L.M. The application of U–Pb geochronology to mafic, ultramafic and alkaline rocks: an evaluation of three mineral standards. Chemical Geology. 2009. V.261. P. 43–52. Hoskin P.W.O., Ireland T.R. Rare earth element chemistry of zircon and its use as a provenance indicator. Geology. 2000. V. 28. No.7. P. 627-630. Kempe U., Götze J. Cathodoluminescence (CL) behaviour and crystal chemistry of apatite from rare-metal deposits. Mineralogical Magazine. 2002. V.66. P. 151–172. Kirnarsky Yu.M. Some peculiarities of distribution and composition of accessory baddeleyite from carbonatites. In: New data about Kola Peninsula minerals. Apatity. 1979. P. 76-82 (in Russian). Klemme S., Meyer H.-P. Trace element partitioning between baddeleyite and carbonatite melt at high pressures and high temperatures. Chemical Geology. 2003. V.199. P. 233–242. Krasnova N.I., Kopylova L.N. The geological basement for mineralogical mapping survey (Kovdor deposit). Reports of Academy of Sciences of USSR. Geology. 1988. №5. P. 81-92. Kopylova L.N., Krasnova N.I., Martovitskaya N.A., Poritskaya L.G. Typical chemical features of calcite and baddeleyite of the Kovdor complex deposit. In: «Alkaline magmatism and apatite potential of the North of Siberia». Proceedings of NIIGA. L-d. 1980. P. 124-138 (in Russian). Landa E.A., Krasnova N.I., Tarnovskaya A.N., Shergina Yu.P. REE and Y distribution in apatite from alkaline-ultramafic massifs and carbonatites and genesis of apatite mineralization. Geochemistry. 1983. №1. P. 91-101. Reischmann T., Brugmann G.E., Jochum K.P., Todt W.A. Trace element and isotopic composition of baddeleyite. Mineral Petrology. 1995. V.53, P. 155–164. Rimskaya-Korsakova O.M., Dinaburg I.B. Baddeleyite in ultrabasic and alkaline massifs of the Kola Peninsula. In: Mineralogy and geochemistry. Issue 1. L-d. 1964. P. 13-30 (in Russian). Rodionov N., Belyatsky B.; Antonov A., Kapitonov I., Sergeev S. Comparative in-situ U-Th-Pb geochronology and trace element composition on baddeleyite and low-U zircon from the carbonatite Paleozoic Kovdor Massif (Kola Peninsula). Gondwana Research. 2012. V.21. P. 728-744. Rubatto D., Scambelluri M. U-Pb dating of magmatic zircon and metamorphic baddeleyite in the Ligurian eclogites (Voltri Massif, Western Alps). Contributions to Mineralogy and Petrology. 2003. V.146. P. 341–355. Xie L.W., Zhang Y.B., Zhang H.H., Sun J.F., Wu F.Y. In situ simultaneous determination of trace elements, U-Pb and Lu-Hf isotopes in zircon and baddeleyite. Chinese Science Bulletin. 2008. V.53. P. 1565–1573. |
|