2012
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Новости |
| Первый циркуляр |
| Второй циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
|
Тезисы международной конференции |
Abstracts of International conference |
|
Experimental studying of carbonatization of silicate melts at partial melting of peridotite Kostyuk, AV, Gorbachev, NS Institute of Experimental Mineralogy RAS, Chernogolovka, Russia
Association of carbonates with silicate glass and sulphides in the metasomatized mantle peridotite and eclogite nodules indicate the presence of alkali-carbonate-sulphide fluids in upper mantle and their important role in mantle metasomatism and in melting of metasomatized mantle [1-3]. The study of the physicochemical conditions of formation of immiscible carbonate, silicate and sulphide melts at melting of the mantle peridotite and distribution of the major macro and microelements between coexisting silicate and carbonate melts is of great scientific interest and belongs to the actual petrology problems. Experimental modelling of silicate-carbonate-sulphide liquid immiscibility of mantle magmas was studied in the presence of alkali carbonates. The use of alkali carbonate (K,Na)2CO3 is due to the important role of the alkali-carbonate fluid in the mantle metasomatism, as well as the close positional and genetic relationship between alkaline rocks and carbonatite complexes (such as, Okorusu (Namibia), Stjernoy (Norway), Phalaborwa (S.Afrika), Fernando de Noronha Island (Brazil), etc.). Phase relations in the system peridotite – alkali carbonates with the addition of accessory minerals (apatite, pyrrhotite, ilmenite, zircon) have been studied experimentally at a pressure of 3.9 GPa and temperature of 1250ºC. Experiments were carried out in the IEM RAS on the "anvil with hole" in Fe-bearing platinum capsules using a quenching technique. The temperature is measured by a Pt30Rh/Pt6/Rh thermocouple. At high temperature, pressure is calibrated using a curve of balance quartz - coesite. Uncertainties are ± 5ºC for temperature and ± 0.1 GPa for pressure measurements. Duration of experiments were from 6 to 8 hours. Products of experiments were studied by PC-controlled scanning electron microscope Tescan VEGA TS 5130MM with detector of secondary and backscattered electron on the YAG-crystals and energy dispersive X-ray microanalyzer with semi-conductor Si(Li) detector INCA Energy 350. The powders of spinel peridotite xenoliths from kimberlite pipes Obnazhennaya [4] used as starting materials. Accessory minerals are apatite, ilmenite, zircon, and pyrrhotite was added to the experiments to determine the distribution coefficients of titanium, phosphorus, sulphur and zirconium between coexisting silicate and carbonate melts. Starting materials were used in the ratio: peridotite (70%) - (K,Na)2CO3 (10%) - ilmenite (5%) - apatite (5%) - zircon (5%) - sulphide (FeS) (5%). At partial (10%) melting of peridotite liquidus association of phlogopite–clinopyroxene–zircon–X-phase (undiagnosed, similar in composition to the Ti–Cpx) cemented by intergranular silicate glass with inclusions of carbonate and sulphide phases (Fig. 1). The morphology, structure and relationships of glass, carbonate and sulphide globules indicate the existence of immiscible silicate, carbonate and sulphide melt under the experimental conditions.
Fig. 1. Micrograph of polished sample after the experiment in the system peridotite - alkali carbonates (T = 1250ºC, P = 3.9 GPa). The sample represented by immiscible silicate (LSil), carbonate (LCarb) and sulphide (LSulph) melts coexisting with clinopyroxene (Cpx) and phlogopite (Phl).
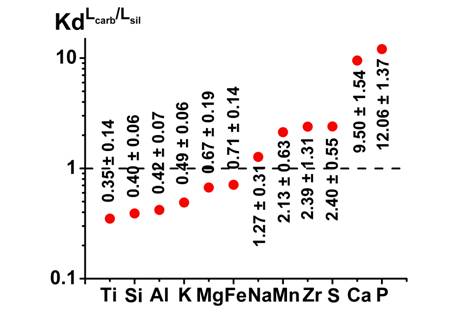
The composition of the silicate melt was phonolites with concentration of sulphur <0.1wt.%. Calcium carbonate melt contained small amounts of alkali metal and silicate components. The solubility of zircon in silicate melt reached 0.70 ± 0.23 wt.% ZrO2, in coexisting carbonate melt 1.28 ± 0.31 wt.% ZrO2. The absence of ilmenite and apatite in the experimental samples can be explained by the high solubility of titanium and phosphorus in the coexisting phases. The concentration of TiO2 in the silicate melt was 1.2 ± 0.35 wt. %, in the carbonate melt 0.42 ± 0.16 wt.%. The concentration of P2O5 was 9.67 ± 1.90 wt.% in the carbonate melt and 2.33 ± 0.31 in the silicate melt. The concentration of sulphur in these melts do not exceed 0.2 wt.%. Thus, in the system peridotite - alkali carbonates, silicate-carbonate-sulphide stratification of alkali silicate melts observed at T = 1250ºC and P = 3.8 GPa. There was a complete dissolution of ilmenite and apatite; silicate, carbonate and sulphide melts coexisting with zircon, phlogopite, and clinopyroxene. The distribution coefficients of macro and microelements (including titanium, zirconium, phosphorus, sulphur) between coexisting silicate and carbonate melts showed that the main concentrator of Na, Mn, Zr, S, Ca, P is the carbonate melt, the main concentrator of Si, Al, K, Mg, Fe, Ti is silicate melt.
Fig. 2. The distribution of the major macro and micro elements between coexisting silicate and carbonate melts.
The data on the distribution coefficients of macro and micronutrients (Ti, Zr, S, P, etc.) between the silicate and carbonate melts and coexisting phases are interesting for the construction of genetic models of carbonate and sulphide magmas and associated deposits of rare elements, platinum-copper sulphide and nickel deposits. Supported by grant RFBR 12-05-00777-a.
References: 1. Kogarko L.N., Henderson C.M.B., Pacheco H. Primary Ca-rich carbonatite magma and carbonate-silicate-sulphide liquid immiscibility in the upper mantle. Contrib. Mineral. Petrol. In 1995. V.121. P. 267-274. 2. Ionov D. Trace element composition of mantle-derived carbonates and coexisting phases in peridotite xenoliths from alkali basalts. J. Petrol. 39. In 1998. P. 1931-1941. 3. Kogapko L.N. Role of deep fluids in the genesis of mantle heterogeneities and alkaline magmatism. Geology and Geophysics, 2005, v. 46, № 12, P. 1234-1245. 4. Ukhanov A.V., Ryabchikov I.D., Kharkiv A.D. Lithospheric mantle of the Yakutian kimberlite province. Moscow. Publishing House "Nauka", 1988. |
|