2011
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Новости |
| Первый циркуляр |
| Второй циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
Тезисы международной конференции
Abstracts of International conference
Experimental modeling of the
alkaline complexes mineralogenesis
Kotelnikov A.R.,
Kovalskii A.M., Kovalskaya T.N., Suk N.I.
Institute of Experimental Mineralogy, Chernogolovka, Russia, tatiana76@iem.ac.ru
Experimental study of the
conditions of mineral genesis of alkaline complexes and application of the
results to natural features is an important task, since such complexes are
associated giant mineral deposits. Our team over the last years studied
solid solutions of alkali pyroxenes, sodalite, thermodynamics
clinopyroxene-biotite paragenesis, fluid regime of formation of post-magmatic
associations. Also, the synthesis of many minerals, typical of alkaline
complexes: sodalite ussingite, cancrinite, alkali pyroxene, gakmanit, etc.
In this study, collected the latest research results.
Association
clinopyroxene-biotite.
Association of clinopyroxene and biotite - one of the most common in
igneous, metamorphic and metasomatic rocks, including rocks of elevated
alkalinity. Clinopyroxene - biotite geothermometer, built on the basis of
empirical data on the composition of natural coexisting Cpx and Bi was
proposed by L.L. Perchuk. However, with regard to alkaline rocks it is
difficult to use due to lack of data on the thermodynamics of alkali
pyroxenes and the influence of alkaline components in the distribution of
magnesium and iron between pyroxene and biotite. As the starting materials
used synthetic solid solutions of ternary clinopyroxenes (CPx-3) and
synthesized at 650 º C and 1.5 kbar phlogopite and annite. Approach to
equilibrium osuschestvyalsya from both sides. Initial compositions of
minerals and the results of experiments on the cation exchange are presented
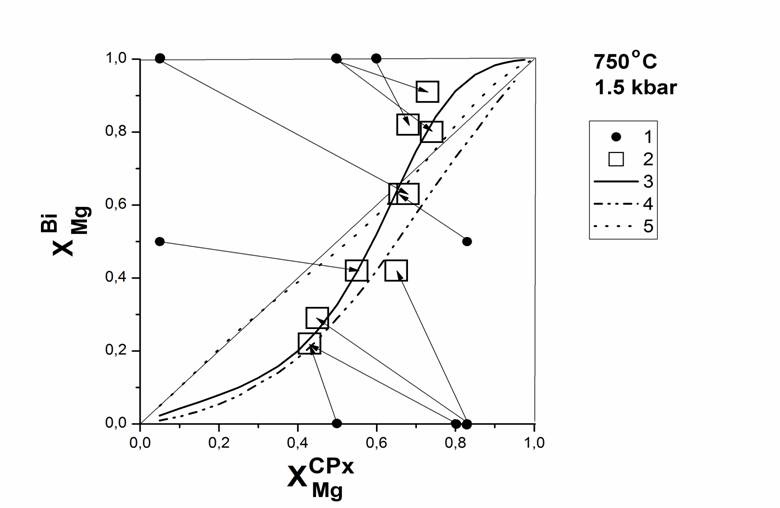
in Table 1. Based on a 10 cation-exchange experiments obtained isotherm
distribution of Mg and Fe between CPx-3, and Bi. (Fig.1). The distribution
coefficient of Mg between clinopyroxene and biotite (KD) is described by the
following equation of third order: ln (KD) = 0.65 + 3.30 * x -5.763 * x2
-1.0911 * x3 (± 0.40), where x - mole fraction of Mg in
clinopyroxene (x = Mg / (Mg + Fe2 +)). According to this equation
to calculate the energy parameters of an asymmetric Margules model to
describe the excess energy of mixing of solid solutions of clinopyroxenes
(system Aeg - Di - Hed; aegirine mole fraction of 0.2 ± 0.04): W1 = -48.5
(16.2) and W2 = 24.1 (2.5) kJ / mol . Previously, we studied the balance of
SPx-Bi for binary solid solutions of clinopyroxene (Kovalskii et al, 2008,
2009) and shows a nearly ideal miscibility in the series diopside -
hedenbergite. Thus, we can conclude that increasing non-ideality of solid
solution of diopside - hedenbergite when entering aegirine end-member. Based
on data Perchuk (Perchuk, 1970) on natural parageneses clinopyroxene and
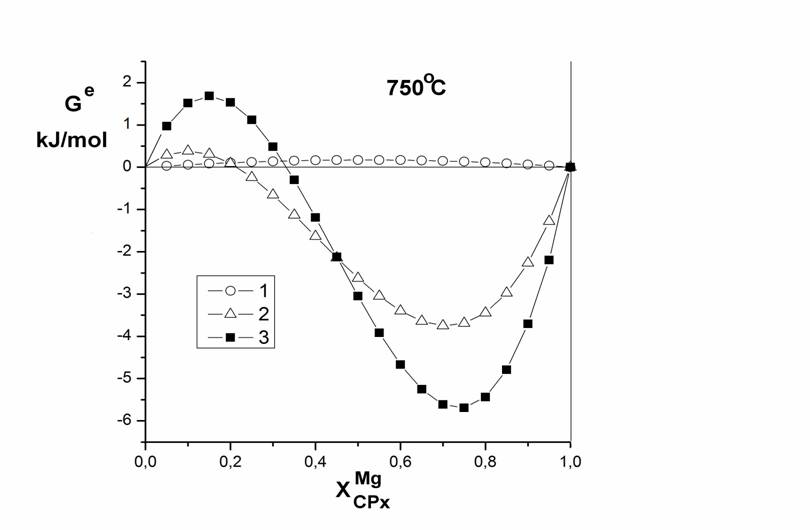
biotite to 750 º C isotherm calculated excess energy of mixing of
clinopyroxenes (Fig. 2).
|
|
|
Fig. 1. Mg, Fe distribution between ternary solid solution of CPx (Aeg-Di-Hed, XAeg=0.2) and biotite. 1– initial compositions of CPx and Bi; 2 – equilibrium compositions of CPx and Bi after experiments; 3 – isotherm of Mg, Fe distribution between CPx and Bi (our data); 4 – isotherm of Mg, Fe distribution between CPx and Bi (natural paragenesis (Perchuk, 1970)); 5 – isotherm of Mg, Fe distribution between binary CPx and Bi (Kovalsky et al, 2008). |
At 750 º C and 1.5 kbar hydrothermal conditions studied the distribution of magnesium and iron between clinopyroxene (ternary alloys of the system Di-Hed-Aeg; XAegCPx = 0.2) and biotite (binary solid solutions Phl-Ann). It is shown that the distribution of Mg, Fe2 + between clinopyroxene and biotite imperfect, at low Mg ferrous iron enriched biotite, with XMgCPx> 0.7 is inverted and Fe2 + is redistributed in the CPx. Based on data on the distribution of Mg, Fe2 + between clinopyroxene and biotite calculated parameters Margules mixing model clinopyroxene, it is shown that the magnitude of the integrated excess mixing energy directly correlates with the mole fraction of the third end-member clinopyroxene (aegirine and jadeite).
|
|
|
Fig. 2. Concentration dependences of excess mixing energies of clinopyroxenes solid solutions. 1 – binary CPx (Di-Hed range); 2 – natural CPx (Perchuk, 1970); 3 – ternary solid solutions of CPx (Aeg-Di-Hed, XAeg=0.2). |
Table 1. The experimental results of Mg and Fe exchange between clinopyroxene (CPx-3) and biotite (Ann – Phl range) at 750oC and 1.5 kbar. KD=[XMgCPx3*(1-XMgBi)]/[(1-XMgCPx3)* XMgBi]
|
№ |
XmgCPx3 until/exp |
XmgBi until/exp |
XmgCPx3 after/exp |
Variation |
XmgBi after/exp |
Variation |
KD |
ln(KD) |
|
6424 |
0.50 |
1.0 |
0.73 |
0.70÷0.75 |
0.91 |
0.90÷0.93 |
0.267 |
-1.319 |
|
6431 |
0.83 |
0.0 |
0.65 |
0.62÷0.66 |
0.42 |
0.40÷0.43 |
2.565 |
0.942 |
|
6433 |
0.80 |
0.0 |
0.43 |
- |
0.22 |
0.20÷0.23 |
2.675 |
0.984 |
|
6489 |
0.83 |
0.5 |
0.66 |
0.63÷0.77 |
0.63 |
0.59÷0.67 |
1.140 |
0.131 |
|
6490 |
0.05 |
1.0 |
0.68 |
0.64÷0.72 |
0.63 |
0.62÷0.63 |
1.248 |
0.221 |
|
6491 |
0.05 |
0.5 |
0.55 |
0.47÷0.55 |
0.42 |
0.42÷0.45 |
1.689 |
0.523 |
|
6492 |
0.60 |
1.0 |
0.68 |
0.65÷0.69 |
0.82 |
0.81÷0.83 |
0.466 |
-0.762 |
|
6499 |
0.50 |
0.00 |
0.43 |
0.41÷0.45 |
0.22 |
0.21÷0.23 |
2.675 |
0.984 |
|
6501 |
0.83 |
0.00 |
0.45 |
0.38÷0.46 |
0.29 |
024÷0.30 |
2.003 |
0.695 |
|
6505 |
0.5 |
1.0 |
0.74 |
0.60÷0.74 |
0.80 |
0.80÷0.82 |
0.711 |
-0.340 |
ln(KD) = 0.65 + 3.30*x -5.763*x2 -1.0911*x3 (±0.40) (1)
Sodalite-bearing association. To date, according to data on the compositions of sodalite and the temperatures of their formation to estimate the minimum concentration of salts (NaCl, Na2SO4) in mineral-fluid: from 10-20 wt.% NaCl-eq. for 2-sodalite paragenesises to 1.5-3 wt.% NaCl-eq. for nozean containing paragenesis. Also estimated the mole fraction of sulfur in the fluid: 0.02 for 2-sodalite paragenesises and 0.04-0.27 for nozean-bearing parageneses (Suk et al, 2007). In the pegmatite body near the mountain Karnasurt found two types of sodalite: chlorine-and sulfur-containing sodalite. The results of our experiments are shown, that heterophase fluid was involved during Lovozersky massif pegmatite formation at 400 - 450°С and homogenius fluid - at 250°С, that correlate with the data Ustinov VI et al. (Ustinov et al, 2006), which is shown in the composition of minerals and their occurrence in the rocks.
Work is supported by RFBR grant № 10-05-00870
References:
Kovalsky A.M., T.N. Kovalskaya, A.R. Kotelnikov (2008) Calibration and application of mineral thermometer based on the study of clinopyroxene-biotite equilibrium. Abstracts of Annual Seminar of Experimental Mineralogy, Petrology and Geochemistry. Moscow, GEOKHI RAS, 22-23 april 2008, pp. 36-37. (in Russian)
Kovalsky A.M., T.N. Kovalskaya, A.R. Kotelnikov (2009) Experimental study of Mg and Fe distribution in the system clinopyroxene-biotite, thermometry of natural paragenesis. Abstracts of Russian youth scientific conference “Minerals: structure, properties, investigation methods”. Miass, IM Ural D. RAS, p. 38. (in Russian)
Perchuk L.L. (1970) Equilibria of rock forming minerals. M., Science, 392 p. (in Russian)
Suk
NI, Kotelnikov AR,
Kovalsky AM
Mineral thermometry
and fluid
composition of sodalite
syenites of the Lovozero
alkaline massif.
Petrology. 2007.
v. 15, № 5, pp.
474-492.
(in Russian)
Ustinov VI,
Grinenko VA,
Kotelnikov AR, Suk
NI, Kovalskaya
TN, Smirnova
EP Thermometry
sodalitsoderzhaschih breed
associations and
Tiksheozerskogo Lovozero
alkaline massif. /
/ Proceedings of the National
Conference of "geochemistry, petrology,
mineralogy and genesis of
alkaline rocks" 18-23
September 2006 Miass. 2006.
s.267-272.
(in Russian)