2011
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Новости |
| Первый циркуляр |
| Второй циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
Тезисы международной конференции
Abstracts of International conference
The influence of the agpaitic index (AI) on the silicate -salt (fluoride, chloride) melt immiscibility
Solovova I.P., Girnis A.V.
Institute of Geology of ore deposits, Mineralogy, Petrology and Geochemistry of RAS. Staromonetny, 35. Moscow. Russia.
e-mail: solovova@igem.ru
We studied silicate melt inclusions in the anorthoclase of pantellerites of the Island of Pantelleria, Italy. They contain daughter crystals of anorthoclase (up to 10 wt% Fe2O3), glass, fine-grained multiphase salt globules and H2O-bearing low-density vapor, in which liquid water was occasionally observed. The composition of salt melt globules are NaCl - CaF2 ± NaF and show only minor admixtures of Mg and Fe. Two groups of globules are clearly distinguished: (1) NaCl-CaF2, and (2) NaF – NaCl - CaF2 with variable Na/Са and Cl/F proportions. Globules of these two types may coexist in a single inclusion, although they were never observed in direct contact. Thus, in one inclusion were examined three globules (NaCl - NaF - CaF2), containing 8, 35 and 99 wt % NaF.
Upon heating, the salt phases were completely melted between 500–800°C. The salt melt gradually dissolved in the silicate melt and completely disappeared at 850–1020°C. Melt inclusions at this moment contain a daughter anorthoclase, silicate and salt melts and the gas bubble. The complete homogenization of the melt inclusions with the dissolution of daughter anorthoclase and vapor bubbles was attained at 1020–1080°C.
The homogeneous melt of inclusions contained up to 72 wt % SiO2 and up to 10 wt % Na2O + K2O (Na/K close to 2.1). Average concentration of Fe2O3 tot is 7.4 wt % (from 50 analyses). These melts are rich in F (up to 0.6 wt %), Cl (up to 1.2 wt %) and H2O (0.3 - 0.93 wt %). SIMS analysis revealed high contents of Zr (up to 4300 ppm), Nb (up to 1400 ppm), and Hf (up to 67 ppm), but only minor Ba (70–740 ppm) and Sr (4.5–38 ppm).
Residual silicate melts coexisting with salt globules and daughter anorthoclase are very distinguished from the homogeneous melt of inclusions. They contain up to 1.3 wt % F, and 2.1 wt % Cl that can be considered as the limit of saturation. The main features of these melts are: 1) reduction of Al2O3 concentration (up to 1 wt %) while increasing Fe2O3 concentration (up to 18 wt %) and 2) the sharp increase of AI index = (Na+K)/Al ratio (up to 14) (Figure). Separate of salt melt, thus, occurs only under the condition AI index markedly above 1.
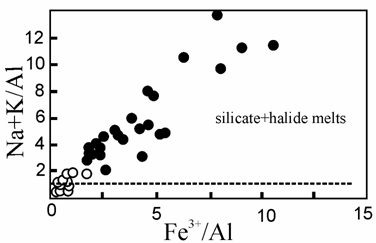
Figure. Correlations between the AI=(Na+K)/Al and Fe3+/Al ratio in the homogeneous (open circles) and residual (solid circles) melt inclusions.
The relationships between the two halide melts remain obscure. The NaCl – NaF - CaF2 system shows no liquid immiscibility field, but the additional components (H2O, SiO2, Al2O3 and, especially, Fe2O3) may affect phase relationships. This problem is a topic of future studies. Our investigation showed that fluoride–chloride melts may separate from peralkaline acid melts containing about 2.1 wt % Cl and 1 wt % F at temperatures about 1000°C.
This study was financially supported by the Russian Foundation for Basic Research and the Russian Program for the Support of Leading Scientific Schools.