2011
| News | Registration | Abstract submission | Deadlines | Excursions | Accommodation | Organizing committee |
| First circular | Second circular | Abstracts | Seminar History | Program | Travel | Contact us |
| Ќовости |
| ѕервый циркул€р |
| ¬торой циркул€р |
| –егистраци€ |
| ќформление тезисов |
| “езисы |
| ѕрограмма |
| ”частники |
| –азмещение |
| Ёкскурсии |
| ѕроезд |
| ¬ажные даты |
| ќргкомитет |
| ќбратна€ св€зь |
“езисы международной конференции
Abstracts of International conference
Geochemistry and petrochemistry of carbonatites and dyke ultrabasites of Chetlassky complex (Timan, Russia)
Nedosekova I.L.*, Udoratina O.V.**, Vladykin N.V.***, Pribavkin S.V.*
*IGG UB RAS Ekaterinburg, Russia; vladi49@yandex.ru
**IG Komi UB RAS, Russia, udoratina@geo.komisc.ru
***IG SB RAS Irkutsk, Russia
The geochemical, petrochemical features and C-O isotope composition of carbonatites and dyke ultrabasites of Chetlassky complex (Timan) have been studied. The petrochemical trends of magmatism of Chetlassky complex were compared with the evolution of hypabyssal alkaline-ultrabasic magmatism in the Ultrabasic Alkaline Complexes (UAC) (on the example of Kolа Alkaline Province); and the geochemical features of carbonatites of Chetlassky complex were compared with the carbonatites of the main formation types of alkaline-ultrabasic magmatism (carbonatite and kimberlite).
The Chetlassky dyke complex of subalkali ultrabasites (picrites and lamprophyres), fenites and carbonatites is located in Middle Timan, in the south-eastern part of Chetlassky Kamen. Dyke bodies of ultrabasites occur in terrigenous rocks of chetlasskaya and bystrinskaya series (R2-R3) and trace faults of north-eastern strike. The ultrabasite dykes are spatially and temporarily related to alkaline metasomatites (fenites, phlogopitic micaites, feldspar metasomatites) and carbonatites with accessory rare earth mineralization Ц pyrochlore, columbite, ilmenorutile, monacite, and also hydrothermal goethite-feldspar and quartz-goethite-hematite rocks (Kostyukhin, Stepanenko, 1987).
The dyke ultrabasites of Chetlassky complex represent holocrystalline rocks with porphyritic and amygdaloid structure containing phenocrysts of olivine (Ol), clinopyroxene (Cpx) Ц augite-Ti-augite, phlogopite (Phl), and also poikilocryst Phl in microlitic Phl-—px basis. The basis also includes carbonate (—arb), sometimes apatite (Apt), garnet (Gr), amphibole (Amf). Together with phenocrysts the rocks show the presence of megacrysts Ol, bright green Cpx и brown-green spinel (Sp), and also xenolites of serpentinites and phenitized gneisses. Accessory minerals of ultrabasites Ц brown Sp, Mgt, Apt, Gr. The amygdales in ultrabasites are composed of Alb-Apt-Gr-Carb aggregate. It should be noted that a diamond finding is known in the Chetlassky lamprophyres (Makeev et al., 2008).
In the thickest dyke bodies (in Kosyu location) in ultrabasites with phenocrysts Ol, Cpx and Phl, and also megacrysts Ol and brown-green Sp, the basis is composed of acicular blue-green Amf, Carb, Apt and tetraferriphlogopite. Phenocrysts Ol are substituted by serpentine and carbonate, and in the edges of Ol grains the margin forms, which is composed of fibrous Amf, tetraferriphlogopite and Mgt. The carbonatite veins with rare metal mineralization are confined to these rocks.
Carbonatites are composed of dolomite, ankerite, calcite and contain micas (Phl -tetraferriphlogopite), amphiboles, aegirine, KFS, albite, sometimes quartz. Carbonatites contain accessory pyrochlore, columbite, monazite, ilmenorutile, bastnesite, baddeleyite, zircon, titanite, apatite, magnetite, ilmenite, barite, torite, orthite.
According to the nomenclature of ultrabasic dyke rocks the Chetlassky dyke ultrabasites are identified as calcium-alkali lamprophyres of spessartite-kersantite range (Makeev et al., 2008). The most magnesial members of Chetlassky dyke ultrabasites represent subalkaline picrites. The major part of Chetlassky rocks is represented by lamprophyres, which in their basis contain carbonate, apatite, amphibole, garnet, oxides, as well as phlogopite and pyroxene. These rocks can be related to ailikite according to classification of ultramafite lamprophyres (Rock, 1986).
The Chetlassky complex differs from autonomous picrite-lamprofiric series by the presence of magnesial varieties of subalkaline picrites, which are petrochemically and geochemically similar to vein kimberlite-picrites. At that Chetlassky lamprophyres correspond to early and middle members of picrite-lamprophyre series, not reaching maximum contents of Al, Ti, Ca, P and alkali, and as a consequence, they donТt contain melilites and feldspatoids, which are characteristic for UAC carbonatite complexes. The low contents of Ti and higher contents of Al should be noted in Timan lamprophyres in comparison to the rocks of corresponding magnesiality from the dyke series of Kola Alkaline Province.
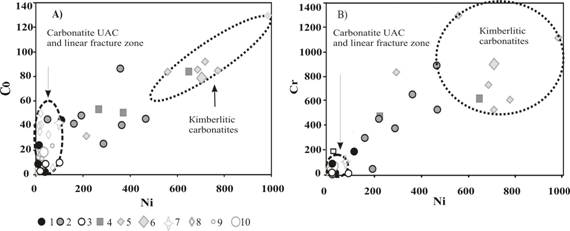
Carbonate-containing lamprophyres (ailikites) of Chetlassky complex are close but are not identical to carbonatites of kimberlite associations (Rock, 1986, Frolov et al., 2005) and also different from classic carbonatites of various formation types (Fig. 1). They are characterized by rather high contents (ppm) of Ni (20-460), Co (7-87), Cr (50-895) and lowered Ba (280-1780), Sr (447-2150), Nb (6-110), REE (175-1340) (Fig. 2 A), compared to the carbonatites of various formation types. They are also characterized by higher ratio Ni/Co (3-11) and lower Nb/Ti (0.005-0.04) compared to carbonatites.
|
|
|
Fig.1. Ni-Co (ј) and Ni-Cr (B) plots for Chetlassky carbonatites. 1-4 Ц rocks of Chetlassky complex: 1Ц REE-carbonatites, 2Цailikites, 3Цcarbonate veins in fenites, 4 Ц picrites and lamprophyres, 5-10 Ц carbonatites of different formation types: 5- kimberlitic carbonatites (Yakutia), 6 Ц average kimberlitic carbonatite; 7 Ц average carbonatites of K-alkaline complexes; 8 Ц carbonatites of Na-alkaline complexes: 8-10 Ц UAC formations, Kola, 9 Ц formations of linear fracture zones,Ilmeno-Vishnevogorsky complex, 10 Ц average magnesiocarbonatite (Wooley, Kempe, 1989). |
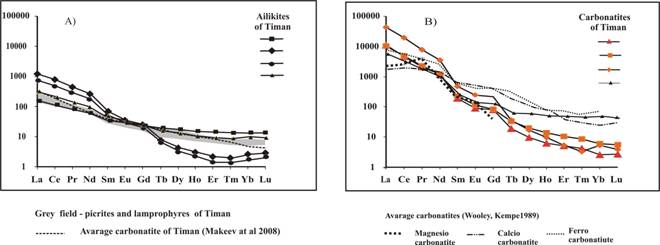
Ankerite-dolomite carbonatites of Chetlassky complex have higher and strongly varying contents (ppm) of Ba (1600-9600), Sr (1060-9700), Nb (до 300), REE (8800-35300) and lower contents of Ni (8-110), Co (1-44), Cr (9-187) compared to ailikites. Carbonatites are also characterized by higher Nb/Ti (0.05-0.09) and lower Ni/Co (0.41-2) compared to lamprophyres and ailikites. On the whole, by its rare element contents the Chetlassky carbonatites are comparable to magnesiocarbonatites of UAC formation varying by very low contents HREE (and accordingly higher La/Yb (6500-35200) and LREE/HREE (150-435)) (Fig. 2 B). It is necessary to note low Sr/Ba (0.4-4) ratio which is characteristic for volcanic and low-abyssal carbonatites (Samoylov, 1984) and also increased contents of Cr, Ni, Co in the Chetlassky carbonatites, what is characteristic for carbonatites is derived from K-ultrabasic picritic-kimberlite magmas (Vladykin, 2009).
|
|
|
Fig.2. Chondrite-nomalized REE pattern of picrites, lamprophyres, ailikites (A) and carbonatites (B) of Chetlassky complex. |
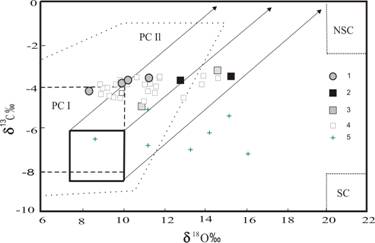
The variations of isotopic composition C and O in the Chetlassky carbonates are within the characteristic ranges for mantle systems and primary magmagenic carbonatites (Yavoy, Pineau, 1986; Ray, Ramesh, 2000) (Fig. 3). The points of C and O isotopes form a trend from ailikites to carbonatites, characterized by weighing δ18ќ (from 8.3 to 15.2 Й) at less significant increase of δ13— (-3.1Е-4.9Й), on the whole corresponding to the trend of fractioning of C and O isotopes during the evolution of carbonate mantle systems.
|
|
|
Fig.3. C and O isotope composition of carbonate from carbonatites, ailikites and picrites of Chetlassky complex. Composition fields: PC I Ц primary carbonatites (Keller, Hoefs, 1995), PC II Ц primary carbonatites (Ray, Ramesh, 2000), NSC Ц marine normal-sedimentary carbonates, SC Ц soil carbonates (Salomons, 1975). The solid bold lines are the mantle square with trends of carbonatite magma differentiation according to (Yavoy, Pineau, 1986). 1Ц ailikites, 2 Ц carbonatites, 3 Ц carbonate veins in fenites, 4 Ц carbonatites according to (Kostyukhin, Stepanenko, 1987), 5 Ц carbonate in bulk in picrites (Kostyukhin, Stepanenko, 1987). |
Conclusions. The comparison of petrochemical trends of magmatism of Chetlassky complex to the petrochemical evolution of hypabyssal (dyke) alkaline-ultrabasic magmatism in the UAC complexes and to the petroevolution of kimberlite magmas showed that dyke ultrabasic magmatism of Timan is close to the early and medium stages of autonomous picrate-lamprophyre series associated with ultrabasic alkaline complexes and varying first of all by the lack of feldspatoids, lower contents of Ti, P and higher Al, and also by the presence of more magnesial varieties of subalkaline picrites close to vein kimberlites. The study of Chetlassky carbonate rocks revealed the presence carbonate-containing rocks of lamprophyre group (ailikites), which are characterized by higher contents of Ni, Co, Cr and lower contents of Ba, Sr, Nb, REE compared to carbonatites. The Chetlassky carbonatites have higher and strongly varying contents of Ba, Sr, Nb, REE and lower Ni, Co, Cr compared to ailikites and lamprophyres, and by their geochemical features they are comparable to classic magnesio- and ferrocarbonatites of different formation types.
This study was financially supported by program of UB, SB and FEB RAS є 09-—-5-10142009-2011.
References:
Vladykin N.V. Potassium alkaline lamproite-carbonatite complexes: petrology, genesis, and ore reserves // Geology and Geophysics. 2009. є50. P. 1Ц10.
Makeev A.B., Lebedev V.A., Bryanchaninova N.I. Magmatites of Middle Timan. Ekaterinburg: UB RAS, 2008. 348 p.
Kostyukhin M.N., Stepanenko V.I. Baikal magmatism of Kanin-Timan region. Leningrad.: Nauka. 1987. 232 p.
Frolov A.A., Lapin A.V., Tolstov A.V. et al. Carbonatites and kimberlites. Moscow: NIA-Priroda, 2005. 540 p.
Rock N.M.S. The nature and origin of Ultramafic Lamprophyres: Alnoites and Allied Rocks // J. Petrology. 1986. є 27. P. 155Ц196.