2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Новости |
| Первый циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
Partitioning of trace elements in diamond-forming carbonatite and ecligite-carbonatite systems in experiments at 7-8.5 GPa
Kuzyura A.V.*, Okoemova V.Yu.**, Litvin Yu.A.*, F.Wall***, T.Heffries****
* Institute of Experimental Mineralogy RAS, Chernogolovka, Russia; ** geological department of Moscow State University, Moscow, Russia; *** Camborne School of Mines, University of Exeter, Cornwall, UK; **** Department of Mineralogy, Natural History Museum, London, UK.
shushkanova@iem.ac.ru
A better understanding of the geochemical behaviour of trace elements at mantle conditions relevant to diamond formation may help to constrain the identity of the mantle medium from which natural diamonds can grow. Garnet and clinopyroxene occur as primary inclusions in diamonds and are part of the peridotite-pyroxenite and eclogite-grosspydite parageneses that crystallize with diamond. Experimental study of trace element partitioning between these minerals and homogeneous completely miscible carbonate-silicate melt from which they would have formed together with diamond has not yet been done but is essential in order to understand the geochemistry of the mantle that is associated with the genesis of diamond.
The goal of the experimental work reported here was to study phase transformations and inter-phase partitioning of trace elements under high pressure melting of a natural silicocarbonatite (Chagatai carbonatite), and also model multicomponent eclogite-carbonatite system ([CPx40-64Grt16-40(SiO2)20]59.3Carb39.3RE1.4) to which a geochemically representative set of trace elements (RE) had been added.
High pressures and temperatures were generated using a toroidal press “anvil-with-hole” with a cell made of lithographic limestone (Litvin et al., 2008) and a tubular heater made of analytical grade graphite. Electron microprobe and SEM examination were carried out in IEM RAS using a CamScan M2300 (VEGA TS 5130MM) equipped with a Link INCA energy-dispersive spectrometer. Contents of trace elements in grains on the same samples (with carbon coating removed) were determined by LA-ICP-MS in the Department of Mineralogy at the Natural History Museum, London. Homogeneous quenched melt areas and isometric grains of garnet and clinopyroxene surrounded by the melt were analyzed. Electron microprobe determinations of Ca were used as an internal standard for correction of the LA-ICP-MS data.
With experimental parameters corresponding to the PT conditions at which diamond is thermodynamically stable, complete or partial melting of starting carbonate-silicate rocks leads to the formation of completely miscible carbonate-silicate melts (Litvin, 2007). An extremely important point is that the Chagatai carbonate-silicate melt quenches to practically homogeneous dense glass (Fig. 1), instead of the fibrous dendrite-like structures often observed in experiments on “carbonate” synthesis of diamond (Shushkanova and Litvin, 2008). This produces large enough areas for the acquisition of representative electron microprobe and LA-ICP-MS data. Another important feature was the crystallization of large (up to 100 µm) garnet and clinopyroxene single crystals of Prp0.4 -1.0Gros2.1-2.3Alm0.1-0.6 and Di0.2–0.3Hed0.2-0.3Jd04-0.6 compositions (Fig. 1). Such kind of quenched melt in eclogite-carbonatite system in syngenesis with garnet and clynopyroxene crystals was obtained in few runs. The most samples were zoned because of temperature gradient in ampoule, but central areas we used for the studying correspond to real experimental conditions.
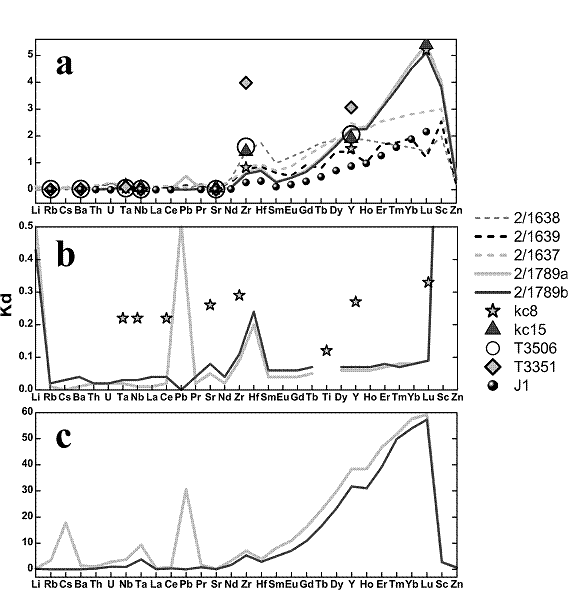
The partition coefficients for Li, Rb, Cs, Ba, Th, U, Ta, Nb, La, Ce, Pb, Pr, Sr, Nd, Zr, Hf, Sm, Eu, Gd, Tb, Dy, Y, Ho, Er, Tm, Yb, Lu, Sc, and Zn were determined. The concentration of trace elements in coexisting garnet, clinopyroxene and carbonate-silicate melt has been measured and the partition coefficients calculated and compared with other carbonatite-mantle mineral element partition studies at lower pressures (Sweeney et al., 1992, 1995).
The garnet/carbonate-silicate melt, clinopyroxene/carbonate-silicate melt, and garnet/clinopyroxene partition coefficients are plotted in Fig. 1 (a, b, c, respectively).
The main feature of the trace element partitioning in our experiments is the different behaviour of light REE (La, Ce, Pr) in relation to medium and heavy REE (Nd, Zr, Hf, Sm, Eu, Gd, Tb, Dy, Y, Ho, Er, Tm, Yb, Lu). Light REE are partitioned favorably into the melt phase, and the other REE go into garnet. Other elements, including LILE (Rb, Sr, Ba), Sc, and also Zn, Ta, Pb, Th, and U have a clear affinity for carbonate-silicate melt.

The similarity in partitioning of trace elements between garnet and carbonate-silicate melt and between garnet and silicate melt makes it difficult to choose between the two melts as potential diamond-forming media if only the garnet is available for measurement of trace element concentration. An unambiguous choice between carbonatite and silicate diamond-forming media cannot be made if based on the detection of trace element contents in syngenetic garnet and clinopyroxene inclusions in natural diamond.
|
|
|
Fig.1 Diagram of trace element partition coefficients between: (a) -garnet and carbonate-silicate melt; (b) – clinopyroxene and carbonate-silicate melt; (c) – garnet and clinopyroxene; kc8, kc15, T3506, T3351, J1-literature data; 2/1638, 2/1639, 2/1637, 2/1789 – our samples |
The results show that the trace element partitioning is not significantly altered by changes in melt composition, with HREE always concentrated similarly in the garnet. Carbonate-silicate melt as a diamond-forming medium and carbonatite or silicate melt equilibrated with mantle silicate minerals behave similarly in respect of trace element distribution.
This study was financially supported by RFBR grant 10-05-00654, 08-05-00110, Grants of the President # MK-4735.2009.5 and MK-4754.2009.5.
References:
Litvin, Yu.A., Litvin, V.Yu. and Kadik, A.A. Experimental characterization of diamond crystallization in melts of mantle silicate-carbonate-carbon systems at 7.0-8.5 GPa // Geochemistry International. 2008. 46. 6. P.531-553.
Litvin Yu.A. High pressure mineralogy of diamond genesis. In: Advances in High-Pressure Mineralogy (Ohtani, E., editor): Geological Society of America Special Paper. 2007. 421. P. 83-103.
Shushkanova, A.V. and Litvin Yu.A. Experimental evidence for liquid immiscibility in the model system CaCO3 – pyrope – pyrrotite at 7.0 GPa: the role of carbonatite and sulfide melts in diamond genesis // The Canadian Mineralogist. 2008. 46. P. 991-1005.
Sweeney, R.J., Green, D.H. and Sie, S.H. Trace and minor element partitioning between garnet and amphibole and carbonatitic melt // Earth and Planetary Science Letters. 1992. 114. № 1-2. P.1-14.
Sweeney, R.J., Prozesky, V. and Przybylowich, W. Selected trace and minor element partitioning between peridotite minerals and carbonatite melts at 18-46 kb pressure // Geochimica et Cosmochimica Acta. 1995. 59. P. 3671-3683.