2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Íîâîñòè |
| Ïåðâûé öèðêóëÿð |
| Ðåãèñòðàöèÿ |
| Îôîðìëåíèå òåçèñîâ |
| Òåçèñû |
| Ïðîãðàììà |
| Ó÷àñòíèêè |
| Ðàçìåùåíèå |
| Ýêñêóðñèè |
| Ïðîåçä |
| Âàæíûå äàòû |
| Îðãêîìèòåò |
| Îáðàòíàÿ ñâÿçü |
Iron redox state in granitoidic melts with different alkali content according to experimental data
Volovetsky M.V.*, Rusakov V.S.**, Lukanin O.A.*, Kargaltsev A.A.*
*Vernadsky Institute of Geochemistry and Analytical Chemistry, Moscow, Russia
**Lomonosov Moscow State University, Moscow, Russia
Redox state of iron in melts depends on a set of internal and external parameters: temperature, oxygen fugacity and melt composition. Knowledge of these parameters gives an opportunity to determine redox conditions during melt forming. Despite this problem were regarded in many previous experimental studies T-fO2 influence on Fe2+/Fe3+ ratio in acid melts is still poorly known [Kilinc et al., 1983; Borisov and Shapkin, 1989 et al.]. In present work results of studying of iron redox-ratio in acid melts are presented. High-temperature furnace with controlled oxygen fugacity was used [Kargal’tsev, 2009].
Two granitic compositions were chosen as initial glasses: 1) granite – I2; 2) pantellerite (alkaline granite) – P9. Chemical composition of initial glasses is presented in Table 1.
Table 1. Chemical composition of initial glasses.
|
Series |
SiO2 |
TiO2 |
Al2O3 |
FeO |
MnO |
MgO |
CaO |
Na2O |
K2O |
Cl |
ZrO2 |
Total |
|
P9 |
68.28 |
0.36 |
7.39 |
7.82 |
0.31 |
0.07 |
0.34 |
6.61 |
4.17 |
0.81 |
0.33 |
96.48 |
|
|
0.43 |
0.04 |
0.08 |
0.34 |
0.08 |
0.01 |
0.02 |
0.17 |
0.12 |
0.04 |
0.05 |
0.43 |
|
I2 |
70.46 |
0.06 |
15.28 |
3.41 |
|
0.14 |
2.06 |
4.07 |
3.95 |
|
|
99.48 |
|
|
1.15 |
0.02 |
0.48 |
0.37 |
|
0.02 |
0.18 |
0.09 |
0.07 |
|
|
0.74 |
Glasses were obtained in a set of experiments under temperature from 1120 to 1420°C and in a wide range of fO2: from 10-0.7 (air) äî 10-13 (buffer IW) bar. Alumina crucibles were used as containers. Microprobe analysis has revealed that chemical composition of melts changes with increasing temperature (in particular Na volatilizes). In this connection it is necessary to note that hatch lines in fig.2 relate to glasses with a bit different chemical composition.
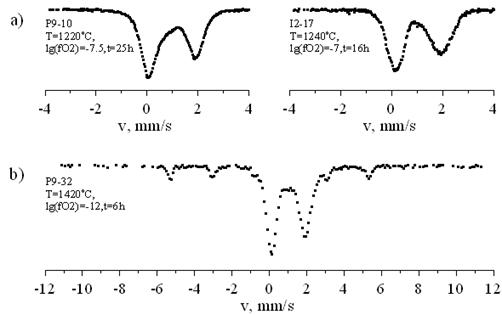
Valent and structural state of iron was investigated using Mössbauer spectroscopy. Mössbauer spectra were recorded under room temperature in absorbance geometry. Experimental spectra can be both paramagnetic (fig1.a.) and with magnetic contribution (fig.1.b.). All the spectra were processed by calculation of few independent hyperfine parameters distribution functions [Rusakov, 2000].
|
|
|
Figure 1. Typical Mössbauer spectra of investigated glasses: a) paramagnetic spectra (glass); b) spectrum with magnetic phase contribution (glass + Fe0). |
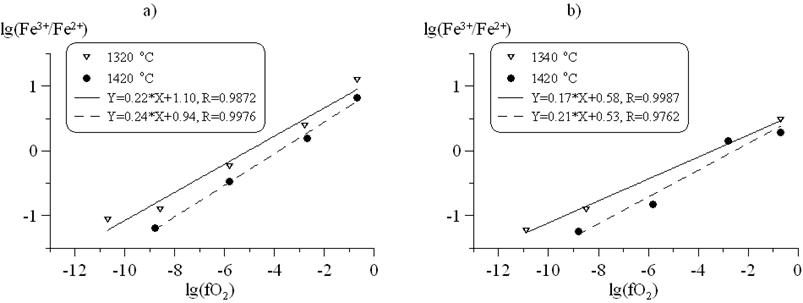
Mössbauer data analysis has shown that iron redox-ratio depends on oxygen fugacity in accordance to the equation lg(Fe3+/Fe2+) = a·lg(fO2) + b(T) (fig.2.) On the figure it is shown that Fe3+/ΣFe ratio in pantelleritic melts is higher than in granitic on 10-20 %. It is also noteworthy that redox-ratio decreases with increasing temperature.
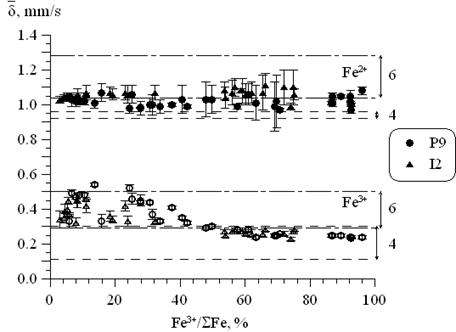
Iron coordination state was determined from average Mössbauer line shifts of corresponding subspectra. Fe3+ ions were revealed to change coordination from octahedral to tetrahedral with increasing of iron redox ratio (beginning from Fe3+/ΣFe>0.6). At the same time a large amount of ions occupy five-fold positions. Fe3+ ions don not change coordination much and occupy five-fold and octahedral positions (fig.3.).
|
|
|
Figure 2. Relationship between logarithms of Fe3+/Fe2+ ratio and fO2 (approximating lines: firm line – 1320ºC (1340ºC), hatch line – 1420ºC). a) pantelleritic composition, b) granitic composition. |
|
|
|
Figure 3. Relation between Mössbauer line shifts and Fe3+/ΣFe ratio. Typical shift ranges of octahedral (6) and tetrahedral (4) positions for mineral systems are shown. |
This study was financially supported by RFBR (grant 08-05-00377) and ONZ RAS (programme 8, 2009)
References:
A.A. Kargal’tsev, M.V. Volovetskii, A.A. Kadik and O.A. Lukanin. A High-temperature furnace with a controlled oxygen regime for studying phase and redox reactions in silicate and oxide systems at 1 Atm // Geochemistry International. 2009. Vol. 47. No. 7. P. 725-730.
Kilinc A., Carmichael I.S.E., Rivers M.L. and Sack R.O. The ferric-ferrous ratio of natural silicate liquids equilibrated in air // Contrib Mineral Petrol. 1983. Vol. 83. P. 136-140.
Borisov A.A., Shapkin A.I. A new empirical equation relating Fe3+/Fe2+ in magmas to their composition, oxygen fugacity, and temperature // Geochemistry International. 1989. Vol. 27. P. 111–116.
Rusakov V.S. Messbauerovskaya spektroskopiya lokal'no neodnorodnykh sistem. Almaty, 2000. 431 p.