2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Íîâîñòè |
| Ïåðâûé öèðêóëÿð |
| Ðåãèñòðàöèÿ |
| Îôîðìëåíèå òåçèñîâ |
| Òåçèñû |
| Ïðîãðàììà |
| Ó÷àñòíèêè |
| Ðàçìåùåíèå |
| Ýêñêóðñèè |
| Ïðîåçä |
| Âàæíûå äàòû |
| Îðãêîìèòåò |
| Îáðàòíàÿ ñâÿçü |
On portlandite from carbonatite complexes
Sokolov S.V.
All-Russian Institute of Míneral Resources (VIMS), Moscow, Russia
vims-sokol@mail.ru
Portlandite Ca(OH)2 is a rare mineral in carbonatite-bearing complexes. There are only a few finds of this mineral in them as solid inclusions in apatite from rocks of the Eastern Sayan complexes, Russia (Podgornykh, 1981), in fluorite from alkaline basaltoids of the Dunkeldyk massif, Tajik Republic (Solovova et al., 1992), in apatite from phoscorites of the Phalaborwa complex, South Africa Republic (Solovova et al., 1998), and in apatites from carbonatites and phoscorites of the Kovdor massif, Kola Peninsula, Russia (our data).
Portlandite was determined by electron microprobe (Table 1, analyses ¹¹ 1 and 6), and also immersion and X-ray phase methods. In all enumerated environments it forms thin, colorless, rounded and ellipse-like segregations or plates, having often hexagonal morphology (Fig. 1); their sizes vary from 20 to 250 µm. Refractive indices of studied portlandites correspond to values no = 1.575-1.576 and ne = 1.543-1.546 (Solovova et al., 1998 and our data).
Other form of occurrence of portlandite within indicated complexes represent daughter phases of inclusions. In such form it was found into primary and pseudosecondary inclusions of saline melt in fluorite and clinopyroxene from basaltoids of the Dunkeldyk massif (Solovova et al., 1992).
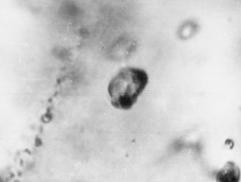
Directly in carbonatites, portlandite daughter phase was established firstly into primary melt inclusions in host-niocalite (Ca,Nb)4[Si2O7](OH,F)2 from monticellite-calcite carbonanite of the Oka massif, Canada (Sokolov, Veksler, 1997). These inclusions had rounded or subprismatic habit and sizes up to 50-60 µm in diameter (Fig. 2). They are contained multiphase microcrystalline aggregate (90-95 vol.%) and interstitial heterogenious (gas + liquid) fluid. An investigation of inclusions through electron microprobes Jeol Superprobe 733 and JXA-8100 Superprobe showed, that daughter phases of diopside, monticellite, calcite, and magnetite are present together with portlandite. Andradite, melilite, phlogopite, apatite, (Na-Ca±K)-carbonate, and (K-Na-Ca)-sulphate-carbonate were determined beside this into other melt inclusions of niocalite (Sokolov, 2007; Sokolov, Veksler, 1997).
I.P. Solovova with colleagues (1998) determined portlandite as daughter phase of fluid-saline inclusion in apatite from phoscorites, which associate with carbonanites at the Phalaborwa complex.
Literary and our data, characterizing the chemical composition of portlandite daughter phases are tabulated in Table 1 (analyses ¹¹ 2-5, 7).
Table 1. Chemical composition of portlandite
|
Massif |
Dunkeldyk |
Oka |
Phalaborwa |
||||
|
Host-mineral |
Fluorite |
Clinopyroxene |
Niocalite |
Apatite |
|||
|
¹¹ analyses |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
|
SiO2 |
|
|
|
1.14 |
1.36 |
|
0.41 |
|
TiO2 |
|
|
|
0.16 |
0.18 |
|
|
|
Al2O3 |
|
|
|
0.18 |
0.09 |
|
|
|
FeOt |
|
|
|
0.19 |
0.28 |
0.03 |
0.14 |
|
MnO |
|
|
|
0.11 |
0.19 |
|
0.27 |
|
MgO |
|
|
|
0.03 |
0.16 |
0.10 |
0.12 |
|
CaO |
72.80 |
75.93 |
74.32 |
70.84 |
68.81 |
73.28 |
80.20 |
|
SrO |
2.60 |
1.04 |
2.66 |
|
|
0.69 |
|
|
BaO |
|
|
|
|
|
0.17 |
|
|
Na2O |
|
|
|
0.41 |
0.53 |
|
|
|
K2O |
|
|
|
0.08 |
0.21 |
|
|
|
P2O5 |
|
|
|
0.01 |
0.01 |
|
|
|
S |
0.00 |
0.00 |
0.01 |
0.07 |
0.09 |
|
|
|
F |
0.00 |
0.00 |
0.01 |
0.00 |
0.00 |
0.00 |
|
|
Total |
75.40 |
76.97 |
77.00 |
73.22 |
71.91 |
74.27 |
81.14 |
|
References |
Solovova et al., 1992 |
Present work |
Solovova et al., 1998 |
||||
Considerably earlier before discovering in carbonatite complexes (as independent mineral or daughter phase of inclusions), portlandite was obtained in laboratory conditions in synthetic systems, more or less close to natural carbonatitic ones. Pioneers results are those of study of system calcite-water, in which portlandite originated as a result of chemical reaction between calcite and water, and at temperatures above 600îÑ existed water-bearing melt of calcite and portlandite (Syromyatnikov, 1958). The author of this work suggested, that rare occurrence of the portladite in nature might be caused by high content of CO2 in mineral forming medium (reaction with which give rise to calcite formation), as well as by presence in them of components, resulting in formation of calcium silicates and, we should add, other Ca-bearing carbonates.
Later P.J. Wyllie and O.F. Tuttle (1960) have received in products of crystallization of melt in system CaO–H2O–CO2 segregations of portlandite, similar by morphology to natural ones. Besides their data supported, that under low ratio of Í2Î/ÑÎ2 in equilibrium fluid it is impossible joint formation of portlandite with calcite.
Experiments in the system CaO–MgO–CO2–H2O showed, that with fractional crystallization the final liquid, taken as synthetic carbonatite melt, would precipitate calcite, dolomite, and portlandite (Wyllie P.J., 1965).
|
|
|
|
Fig. 1. Portlandite solid inclusions in apatite from calcite carbonatite, the Kovdor massif. x 50. |
Fig. 2. Melt inclusion in niocalite from calcite carbonanite, the Oka massif. x 400. |
The data exposed above and followed further support possibility of magmatic origin of portlandite and allow us to assess the temperatures of its formation in carbonatite process: 1) apatite from carbonatites of the Kovdor massif, containing solid inclusions of portlandite, crystallized at 620-750îÑ; 2) inclusions in niocalite from carbonanite of the Oka massif homogenized at 850-890îÑ (Sokolov, 1994); in the process of their heatig dissolution of the portlandite and calcite crystallites began at 450-480îÑ and ended at about 600îÑ; 3) in the system of calcite-water the temperature of potlandite melting was 635-715îÑ under the pressure of 0.5-1.0 kbar (Syromyatnikov, 1958); increasing of Ðfl up to 2 kbar resulted in decreasing of stability temperature of mineral to 615îÑ (Wyllie, 1965).
References:
Podgornykh, N. Inclusions in apatite from the Eastern Sayan carbonatite complexes and their petrological significance / Petrology of lithosphere and ore-bearing. Leningrad, 1981. P. 139 (in Russia).
Sokolov S.V. Formation conditions of the Oka carbonatites // Waterloo`94. GAC-MAC Joint Annual Meeting. Abstracts. 1994.
Sokolov S.V. Phase composition of melt inclusions in monticellite and niocalite from carbonatites of the Oka complex (Quebec, Canada): confirmation of silicate–carbonate liquid immiscibility // ECROFI-XIX. University of Bern, Switzerland, 17-20 July. Abstract Volume. 2007. P. 202.
Sokolov S.V., Veksler I.V. Mineralogy of melt inclusions in niocalite from carbonatites of the Oka complex, Canada // Ottawa’97. GAC-MAC Joint Annual Meeting. Abstracts Volume. 1997. P. A-140.
Solovova I.P., Girnis A.V., Guzhova A.V., Naumov V.B. The magmatic salt inclusions in minerals from the East Pamirs alkaline basalts // Geokhimia. 1992. ¹ 1. P. 68-77 (in Russia).
Solovova, I.P., Ryabchikov I.D., Kogarko L.N., N.N. Kononkova Inclusions in minerals of the Phalaborwa complex, South Africa // Geokhimia. 1998. ¹ 5. P. 435-447 (in Russia).
Syromyatnikov F.V. Materials for study of the system calcite–water / Transactions of the V Conference on experimental and technical mineralogy and petrography. Moscow, Published by the USSR Academy of Sciences, 1958. P. 221-229 (in Russia).
Wyllie P.J. Melting relationships in the system CaO–MgO–CO2–H2O, with petrological applications // Journ. Petrol. 1965. Vol. 6. ¹ 1. P. 101-123.
Wyllie P.J., Tuttle O.F. The system CaO–CO2–H2O and the origin of carbonatites // Journ. Petrol. 1960. Vol. 1. ¹ 1. P. 1-46.