2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Íîâîñòè |
| Ïåðâûé öèðêóëÿð |
| Ðåãèñòðàöèÿ |
| Îôîðìëåíèå òåçèñîâ |
| Òåçèñû |
| Ïðîãðàììà |
| Ó÷àñòíèêè |
| Ðàçìåùåíèå |
| Ýêñêóðñèè |
| Ïðîåçä |
| Âàæíûå äàòû |
| Îðãêîìèòåò |
| Îáðàòíàÿ ñâÿçü |
Composition peculiarities of the altered kimberlites of the International pipe.
Minin V. A.*, Tolstov A.V. **
*Institute of Mineralogy and Petrography SB RAS, Novosibirsk, Russia ** Botuobinskaya Geological Survey Expedition of Joint-Stock Company ALROSA, Mirny, Russia
The International pipe is confined to the Mirny field of the Botuobinsko-Markhinskaya zone of Yakutian diamondiferous kimberlites. The pipe is a cylindrical body with slightly pronounced “socket” which disappears just below 100-120 m. Kimberlite breccias dominate over porphyric kimberlites in the upper horizons, however porphyric kimberlites make up the whole diatreme volume below 370 m. Kimberlites intrude carbonate-terrigenous and salt deposits of the Lower Ordovician-Cambrian. Aggregates of halite crystals occur everywhere in the pipe rocks besides the association of minerals which formed during serpentinization. This report discusses the manifestations of these two types of post-magmatic changes and their influence on the kimberlite composition.
Halite forms in kimberlite irregular shaped, micron sized accumulations confined to the porous spaces in the serpentine-carbonate binder. Its amounts vary from 0.0 n to 3-5%. Na2O contents also change within close limits. On this basis it may be suggested that the whole Na in kimberlite is bonded in halite structure. The absence of Na2O correlation with other main oxides supports its halite nature [Vasilenko et al., 1997]. Both this fact and halite veins, which occur universally in kimberlites, make it possible to consider the solutions penetrating into the pipe from the host salt-bearing bulk to be the sodium source. Judging from the bitumen inclusions in halite crystals the temperature of the solution didn’t exceed 100-300oC. At the same time, Cl-enriched high temperature solutions are, as known, a favorable environment for formation and migration of REE chloride complexes [Haas et al., 1995]. Penetrating into kimberlites or being their derivatives high temperature thermal springs must participate in the redistribution of lanthanides. In this case, Na as Cl associated element must show stable correlations with REE. The analysis has demonstrated that statistically significant relations between Na2O and REE are absent. We found similar regularity in the halite-containing kimberlite of the Udachnaya-West and Udachnaya-East pipes. Admittedly, low temperature chloride solutions, which have penetrated from the host salt-bearing deposits, are responsible for halite mineralization in kimberlites.
Detailed studies demonstrate that olivine-serpentine replacement doesn’t occur with brucite formation – four serpentine cells replace nine olivine cells under the action of water fluid: [9Mg8Si4O16]®[4Mg12Si8O20(OH)16]. In this case a significant part of Mg and a part of Si pass into the solution. Thermodynamic calculations are evidence that Mg and Si released during serpentinization may amount to 18 and 7 wt%, correspondingly [Marshintsev, 2005]. Mg- and Si-enriched fluid takes metasomatic effect on the parent rock. In the International kimberlites silica excess is found in talk according to the known reaction: Mg3Si2O5(OH)4 + 1.16SiO2 ® 0.79Mg3Si4O10(OH)2 + 0.63MgO + 1.2H2O. At the first stages of the process talc forms margins, small flaky accumulations around serpentine pseudomorphs after olivine. Further, as the process develops talc aggregates extend over the whole rock volume completely replacing serpentine aggregates. The newly formed quartz is found in some altered kimberlite samples. The study of cores from the exploratory wells shows that in the International pipe altered (silicified) kimberlites make up different in size (from the first cm to the first tens of m) and shape zones. They occur nearly at all depths – from 10-15 to 800 m, and show gradation with unaltered kimberlites.
The main trend of change of kimberlite chemical composition during these transformations is the increase of SiO2 as magnesia concentrations decrease (Table 1).
Table 1. Average compositions of unaltered (1) and altered kimberlites from the International pipe.
|
|
SiO2 |
TiO2 |
Al2O3 |
Fe2O3 |
MnO |
MgO |
CaO |
Na2O |
K2O |
P2O5 |
LOI |
|
1 (n=256) |
29.61 |
0.37 |
2.56 |
5.77 |
0.12 |
30.98 |
5.44 |
1.93 |
1.09 |
0.37 |
21.73 |
|
2 (n=25) |
38.62 |
0.35 |
4.73 |
5.60 |
0.12 |
25.16 |
5.43 |
0.81 |
1.40 |
0.26 |
17.48 |
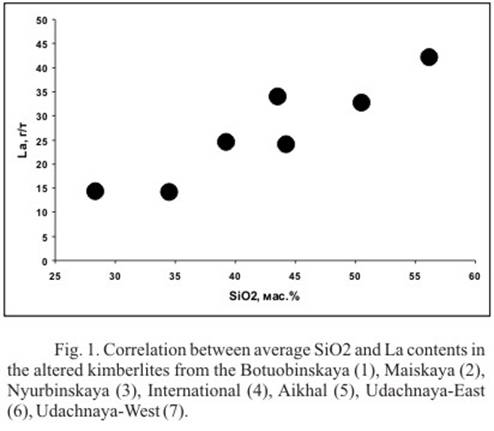
LREE contents in the altered kimberlites decrease more than twofold relative to the unaltered kimberlites (Table 2). Similar tendency is traced in the altered rocks of the Aikhal, Udachnaya-East, Udachnaya-West and Botuobinskaya pipes. In this case SiO2 forms positive regressions with La, Ce, Nd in the altered kimberlites of the enumerated pipes (Fig. 1). Linear pattern of the regressive bands and uniform distribution of figurative dots testify that migration of petrogenous and rare earth elements is caused by a single hydrothermal process. The question of the nature of hydrothermal solutions is still an open question. However the fact that altered kimberlites, collected from different pipes, kimberlite fields and different depths form complementary sequence in the sign space, suggests that kimberlite melts themselves make a major contribution to the formation of solutions.
Table 2. REE contents in the unaltered (1) and altered (2) kimberlites from the International pipe.
|
|
La |
Ce |
Nd |
Sm |
Eu |
Gd |
Tb |
Yb |
Lu |
|
1 (n=49) |
85.0 164-53 |
149.3 294-83 |
54.1 104-27 |
9.9 16.2-4 |
2.1 3-1 |
5.0 9.7-3 |
0.6 1.2-0.4 |
0.6 1.1-0.4 |
0.1 0.1-0.01 |
|
2 |
34.0 |
57.0 |
20.0 |
3.4 |
0.9 |
2.5 |
0.3 |
0.6 |
0.1 |
|
|
Pyropes are the minerals concentrating Gd, Tb, Ya and Lu in kimberlites. In this case the value of LREE/HREE ratio directly correlates with chrome content [Sobolev et al., 1997]. Chromium varieties of garnets, in turn, are thermodynamically the least stable in the near-surface conditions and therefore most subject to hydrothermal effects. Pyropes in altered and unaltered kimberlites of the pipe do not differ in the contents of the main components (Table 3). Therefore they were not involved in the process of hydrothermal conversion. This conclusion is supported by similar contents of HREE in the rocks (Table 2).
Table 3. Average compositions of pyropes from the International pipe unaltered (1) and altered (2) kimberlites.
|
|
SiO2 |
TiO2 |
Al2O3 |
Cr2O3 |
MnO |
MgO |
CaO |
Na2O |
FeO |
Total |
|
1 (n=126) |
41,67 |
0,19 |
19,08 |
5,63 |
0,41 |
19,88 |
4,88 |
0,05 |
8,00 |
99,79 |
|
2 (n=109) |
41,55 |
0,20 |
18,68 |
6,03 |
0,47 |
19,65 |
5,27 |
0,06 |
7,81 |
99,71 |
Refrences:
Vasilenko V.B., Zinchuk N.N., Kuznetsova L.G. Petrochemical models of Yakutian diamond deposits // “Nauka” Siberian enterprise of RAS, Novosibirsk, 1997, 569.
Doroshev A.M., Brey G.P., Girnis A.V., Turkin A.I., Kogarko L.N. Pirope-knorringite garnets in the earths mantle: experiments in the MgO-Al2O3-SiO2-Cr2O3 system// Russian Geology and Geophysics, 1997, vol. 38, no. 2, p. 523-545.
Marshintsev V.K. Vertical zonality of kimberlite bodies (by the example of the Udachnaya pipe studies, Yakutia). The geology of diamonds. Its present and future. - Voronezh: Voronezh State Univ., 2005, p. 847-856.
Sobolev V.N., Taylor L.A., Snyder G.A., Sobolev N.V., Pokhilenko N.P., Kharkiv A.D. A unique metasomatized peridotite xenolith from the Mir kimberlite pipe// Russian Geology and Geophysics, 1997, vol. 38, no. 1, p. 206-215.
Haas J.R., Shock E.L., Sassani D.C. Rare earth elements in hydrothermal systems: estimates of standard partial molal thermodynamic properties of aqueous complexes of rare earth elements at high pressures and temperatures // Geochim. Cosmochim Acta. – 1995. Vol. 59. – No. 21. – P. 4329-4350.