2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| Second circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Новости |
| Первый циркуляр |
| Второй циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
Elpidite: structural transformations on dehydration
Zubkova N.V., Ksenofontov D.A., Chukanov N.V., Nedelko V.V., Pekov I.V., Kabalov Yu.K., Pushcharovsky D.Yu.
Geology Dept., Moscow State University, Moscow, Russia
Institute of Problems of Chemical Physics, of RAS, Chernogolovka, Russia
Institute of Geochemistry and Analytical Chemistry of RAS, Moscow, Russia
ksenofant@rambler.ru
Microporous silicates with heteropolyhedral frameworks, especially titano- and zirconosilicates, attract the attention because of their technologically important properties (ion-exchange, sorption, catalytic, etc.). All these minerals (as well as their synthetic analogues) can be considered as heterosilicates with pronounced zeolite properties (Chukanov and Pekov, 2004). One of the brightest representatives of the natural zirconosilicates of this type is elpidite, Na2ZrSi6O15·3H2O, that is characterized by strong cation-exchange properties. Potentially industrial amounts of elpidite are related to alkaline granites. The richest deposits of the mineral were found at magmatic complexes Khan-Bogdo and Khaldzan-Buregteg in Mongolia (Solodov et al., 1991).
We have studied structural transformations of elpidite from Mt. Alluaiv in the Lovozero alkaline complex (Kola Peninsula, Russia) accompanying its dehydration on heating. The empirical formula of the initial sample calculated for 15 O atoms is: (Na1.98K0.01)(Zr1.02Nb0.03Hf0.01)(Si5.92Al0.02)O15·3.28H2O. The thermal dehydration of elpidite was carried out in the argon atmosphere. THe IR-spectrum of the fully dehydrated sample showed the absence of the bands of OH-groups and H2O molecules (regions of 3000-4000 and 1500-1700 cm-1). Powder X-ray diffraction data for both initial and dehydrated samples were collected using a computer-controlled STOE STADI MP diffractometer (CuKa1 radiation, l = 1.54056 Å). Scan ranges of 4.00 £ 2q £ 109.82o were measured using a STOE linear position sensitive detector. Data treatment and the Rietveld structure analysis were carried out using the Wyriet package of computer programs (Schneider 1989). The profiles were modeled using a Pearson VII function. Unit cell dimensions of the initial sample (a = 7.1136(1), b = 14.6764(2), c = 14.5977(2) Å) are close to those found for elpidite by Cannillo et al. (1973) and this structural model was used as the starting one for the refinement in space group Pbcm. Analysis of the powder X-ray diffraction data of the dehydrated elpidite showed several additional reflections in comparison with the initial elpidite and allowed to reveal the orthorhombic unit cell with a = 14.0899(1), b = 14.4983(1), c = 14.3490(1) Å. Close values of the unit cell parameters and sp. gr. Cmce were recently revealed by us for K- and Rb-exchanged forms of elpidite. Thus for the refinement we used the coordinates of the framework atoms from the model of Rb-exchanged elpidite. Na cations were localized in the larger unit cell according to the model of the initial elpidite and the sites of H2O molecules are vacant. Final agreement factors for initial and dehydrated (in paranthes) elpidite are: Rp = 0.0463 (0.0574), Rwp = 0.0609 (0.0748), RB = 0.0293 (0.0361), RF = 0.0366 (0.0399).
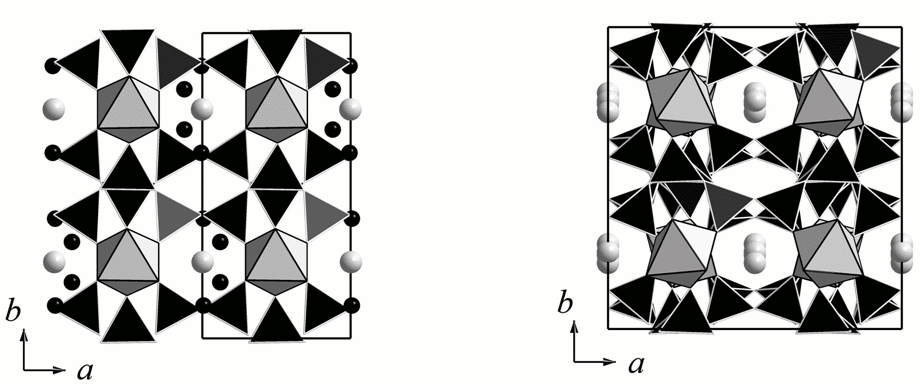
The main structural unit of elpidite is heteropoplyhedral framework described by Cannillo et al. (1973) and formed by double silicate chains connected to each other via isolated ZrO6 octahedra. Na cations and H2O molecules occur in the cavities of the framework (Fig. 1a). Dehydrated elpidite retains the heteroplyhedral Si,Zr,O framework but in its structure the framework is characterized by significant distortions (Fig. 1b) that lead to the doubling of a parameter of the unit cell. Positions of Na cations are close to those found in the initial sample but one of two nonequivalent Na-sites is split onto two subsites with the occupancy of 50% each. The dehydration of elpidite is accompanied by the decrease of the volume Vdehydrated < 2 ´ Vinitial.
 |
|
|
a |
b |
Fig. 1. Crystal structure of the initial elpidite (a) and its dehydrated product (b). ZrO6 octahedra are light-gray, SiO4 tetrahedra are black. Na cations are shown as gray circles, H2O molecules – as black circles.
This study was financially supported by RFBR: grants 09-05-00143-а and 09-05-12001-ofi_m and grants of President of Russian Federation MK-320.2010.5, NSh-4034.2010.5 and NSh-3848.2010.5.
References:
Cannillo E., Rossi G., Ungaretti L. The crystal structure of elpidite // Amer. Miner. 1973. V. 58. Pp. 106-109.
Chukanov N.V., Pekov I.V. Heterosilicates with tetrahedral-octahedral frameworks: Mineralogical and crystal-chemical aspects // Reviews in Mineralogy and Geochemistry. 2005. Vol. 57: Micro-and mesoporous mineral phases. Editors: G. Ferraris & S. Merlino. Pp. 105-143.
Schneider J. Profile refinement on IBM-PC’s, Int. Workshop on the Rietveld method. Petten. 1989. 71 pp.
Solodov N.A., Usova T.Yu., Osokin E.D., Pavlova V.N., Semenov E.I., Skosyreva M.V., Solodova Yu.P., Torikova M.V., Tsyganov A.E. Non-Traditional Types of Rare-Metal Mineral Resources. Nedra, Moscow, 1991. 248 pp.