2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Íîâîñòè |
| Ïåðâûé öèðêóëÿð |
| Ðåãèñòðàöèÿ |
| Îôîðìëåíèå òåçèñîâ |
| Òåçèñû |
| Ïðîãðàììà |
| Ó÷àñòíèêè |
| Ðàçìåùåíèå |
| Ýêñêóðñèè |
| Ïðîåçä |
| Âàæíûå äàòû |
| Îðãêîìèòåò |
| Îáðàòíàÿ ñâÿçü |
Mineral composition of silicocarbonatites from Fuertaventura Island.
Groznova M.V., Kogarko L.N.. Senin V.G.
Vernadsky Institute of Geochemistry and Analytical Chemistry RAS,Moscow alkaline@geokhi.ru
Carbonatites of Fuertaventura island was researched. Carbonatites are submitted as sövites and silicocarbonatites. Carbonatites presented everywhere by dikes up to meter by thickness, or small local weights - shapeless formations up to 2-3 meters thickness, or the most thin veins of 1-5 sm thickness, chaotically located in «Basal complex» islands.
Prominent feature of these silicocarbonatites rocks in difference of sövites is raised contents > 20 % on weight SiO2. Part of silicates of these rocks contains albite, stronalsite, a granate - andradite, pyroxene - aegirite-augite, mica ferruterous phlogopite, sphene, zircon. Part of carbonate presented by calcite and strontic calcite. Also at these rocks there is a graphite, phosphates of the rare grounds (La- Ce- Nd- Sm- Pr), alumosilicate La- Ce-Nd- Sm- Pr, barite, magnetite, ilmenite and wüstite.
Identification of structures of minerals was made by a method of roentgenospectral microanalysis on a spectrometer “Camebax-SX 100”. Results of analyses of minerals are counted on mineral formulas (table 1). Graphite was defined qualitatively in raster radiation of carbon (photo 1). For the first time in carbonatites of Fuerteventura it was founded stronalsite -a mineral from group of firm solutions stronalsite-banalsite. These minerals are isostructural, also they form some continuous mutual transitions due to full isomorphism Ba and Sr.
Wüstite is the indicator of regenerative mode of environment minerogenesis of silicocarbonatites .Wüstite is stable at temperature above 600˚Ñ. Rather high activity of the strongest reducers are necessary for formation of wüstite in natural processes minerogenesis (H2, CO, CH4 and other hydrocarbons). This fact speaks about low fugacity of oxygen.
Wüstite in rock forms fine (up to 1 mm) allocation of spheroidal forms. Frequently meets in joints with ilmenite. In the wustite there are impurity Ti (0,34 %-TiO2),
Mn (0,28 %-MnO), Mg (0,1-0,2 %-MgO), Si (0,71-1,2 % SiO2), P (0,13-0,25 %-P2O5).
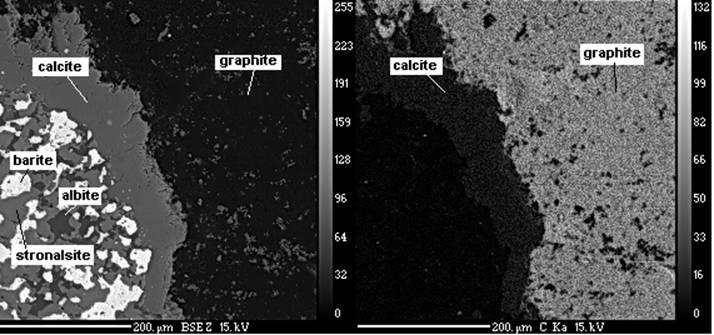
Photo 1.Left- Back-scattered electron images showing relationships between minerals of silicocarbonatite : graphite, calcite, barite,albite, stronalsite. Right- raster radiation of carbon.
Table 1. Microbrobe analyses of albite, garnet, ilmenite, mica, titanite, wüstite, pyroxene.
|
|
albite |
garnet |
ilmenite |
stronalsite |
mica |
titanite |
wustite |
piroxene |
|
SiO2 |
68.96 |
38.03 |
0.05 |
38.71 |
37.73 |
30.33 |
0.71 |
51.64 |
|
TiO2 |
|
0.04 |
51.19 |
0.06 |
3.21 |
35.75 |
0.34 |
0.35 |
|
Al2O3 |
18.73 |
21.11 |
0.1 |
32.07 |
12.02 |
2.32 |
0.21 |
1.22 |
|
FeO |
0.11 |
1.01 |
35.9 |
0.14 |
15.29 |
1.20 |
92.65 |
20.02 |
|
MnO |
|
0.15 |
11.79 |
|
0.35 |
0.07 |
0.26 |
0.74 |
|
MgO |
0.02 |
0.07 |
0.12 |
|
15.77 |
0.01 |
0.14 |
5.55 |
|
CaO |
0.09 |
37.02 |
0.11 |
0.02 |
0.04 |
28.83 |
0.1 |
14.34 |
|
Na2O |
11.48 |
|
|
9.84 |
0.26 |
0.06 |
|
5.89 |
|
K2O |
0.21 |
0.01 |
0.04 |
0.03 |
9.8 |
|
0.02 |
|
|
SrO |
0.02 |
|
|
14.51 |
|
|
|
0.01 |
|
BaO |
|
|
0.03 |
3.21 |
|
|
|
0.03 |
|
P2O5 |
0.15 |
0.17 |
0.21 |
0.22 |
0.14 |
|
0.25 |
|
|
Cl |
0.04 |
|
0.04 |
|
|
|
|
|
|
Totals |
99.8 |
97.62 |
99.57 |
98.81 |
94.59 |
98.57 |
94.81 |
99.78 |
|
|
Cations |
Cations |
Cations |
Cations |
Cations |
Cations |
Cations |
Cations |
|
|
p.f.u. |
p.f.u. |
p.f.u. |
p.f.u. |
p.f.u. |
p.f.u. |
p.f.u. |
p.f.u. |
|
Si |
3.023 |
2.960 |
0.001 |
4.032 |
2.869 |
1.007 |
0.009 |
2.029 |
|
Ti |
|
0.002 |
0.982 |
0.005 |
0.184 |
0.893 |
0.003 |
0.010 |
|
Al |
0.968 |
1.937 |
0.003 |
3.939 |
1.077 |
0.091 |
0.003 |
0.057 |
|
Fe |
0.004 |
0.066 |
0.766 |
0.012 |
0.972 |
0.033 |
0.963 |
0.658 |
|
Mn |
|
0.010 |
0.255 |
|
0.023 |
0.002 |
0.003 |
0.025 |
|
Mg |
0.001 |
0.008 |
0.005 |
|
1.787 |
0.000 |
0.003 |
0.325 |
|
Ca |
0.004 |
3.087 |
0.003 |
0.002 |
0.003 |
1.026 |
0.002 |
0.604 |
|
Na |
0.976 |
|
|
1.988 |
0.038 |
0.004 |
0.002 |
0.403 |
|
K |
0.012 |
0.001 |
0.001 |
0.004 |
0.951 |
|
0.000 |
|
|
Sr |
|
|
|
0.877 |
|
|
|
0.000 |
|
Ba |
|
|
|
0.131 |
|
|
|
0.000 |
|
Totals |
4.987 |
8.070 |
2.016 |
10.989 |
7.904 |
3.056 |
0.988 |
4.111 |
References:
1) Mercedes Munoz, Juana Sagredo, Cristina de Ignacioc, Javier Fernandez-Suareza, Teresa E. Jeffriesd. New data (U–Pb, K–Ar) on the geochronology of the alkaline-carbonatitic association of Fuerteventura, Canary Islands, Spain.// Lithos 85 (2005) 140–153.
2) E. Ancochea, J.L. Brindle, C.R. Cubas, F. Hemiin, M.J. Huertas. Volcanic complexes in the eastern ridge of the Canary Islands: the Miocene activity of the island of Fuerteventura //Journal of Volcanology and Geothermal Research 70 (19%) 183-204.
3) A. Ahijadoa, R. Casillasa, A. HernaÂndez-Pachecob. The dyke swarms of the Amanay Massif, Fuerteventura,Canary Islands (Spain)//Journal of Asian Earth Sciences 19 (2001) 333-345.
4) 4TH EUROCARB WORKSHOP CANARY ISLANDS, SPAIN // 16th-21st September 2003.
5) K. Balogh , A. Ahijado , R. Casillas , C. Fernandez Contributions to the chronology of the Basal Complex of Fuerteventura, Canary Islands//Journal of Volcanology and Geothermal Research 90 1999 81–101.
6) Kaj Hoernle, George Tilton, Mike J., Le Bas Svend Duggen Dieter Garbe-Schonberg. Geochemistry of oceanic carbonatites compared with continental carbonatites: mantle recycling of oceanic crustal carbonate//Contrib Mineral Petrol (2002) 142: 520–542.
7) Koneva M.A. Banalsite and stronalsite from pyroxenites of Zhidoisky massif.//Proceedings of the Russian mineralogical society. ¹ 2-1996. p-103-105.
8) Shumilova Ò.G. A find of diamonds ang graphite-like substances in Fuertaventura carbonatites, Spain // Vestnik Institute of Geology, Komi SC UrO RAS. Syktyvkar, 2005. ¹ 10. P. 17—18. (In Russian).