2010
| News | Registration | Abstracts | Accommodation | Excursions | Deadlines | Organizing committee |
| First circular | Participants | Abstract submission | Travel | Program | Seminar History | Contact us |
| Новости |
| Первый циркуляр |
| Регистрация |
| Оформление тезисов |
| Тезисы |
| Программа |
| Участники |
| Размещение |
| Экскурсии |
| Проезд |
| Важные даты |
| Оргкомитет |
| Обратная связь |
Synthetic analogues of hydrotalcite and the possibility of their use.
Gredina I.V.*, Kulyukhin S.A.**, Krasavina E.P**
*Vernadsky Institute of geochemistry and analytical chemistry, Moscow, Russia; ** Frumkin Institute of physical chemistry and electrochemistry, Moscow, Russia
gredinaiv@gmail.com
Lately people have on the nature of adverse effects and produce changes in the natural radiation background: removed a large quantity of radionuclides in passing the minerals (coal, oil, phosphates, etc.) is the global contamination of the surface of the Earth with radionuclides due to nuclear weapons tests and the Chernobyl accident; allowed local pollution of the environment around the radiochemical plants, nuclear power plants, uranium mines, factories for processing uranium ore, etc. One of the most pressing ecological problems in the field of industrial use of nuclear energy is clean large amounts of liquid radioactive waste (LRW), obtained in the operation of nuclear power plants for various purposes, with the reprocessing of spent nuclear fuel (SNF) in the disposal of the various devices and equipment, contaminated radioactive substances, as well as in solving other technical problems associated with the use of radioactive radiation. To remove radionuclides from liquid radioactive wastes are widely used various adsorbents such as natural (peat, sand, tripoli, zeolites, different coals, minerals, etc.), and synthetic (organic ion exchange resins, polymers, silica gel impregnated with various metals and zeolites, inorganic salts, etc.) . In the process of LRW are used more and more inorganic sorbents. They, unlike organic ion exchangers have a higher mechanical strength, chemical and radiation resistance, many have limited value. After use in the processes of cleaning them with radionuclide accumulation can be sent for disposal as solid radioactive waste in the various matrices (cement, asphalt, glass).
Despite the widespread use of various sorbents in the extraction of radionuclides from liquid radioactive waste, there is no universal sorbent, which allows the simultaneous extraction of the radionuclides present in the solution in the form of cations and anions. For each chemical form are used either cation-exchange or anion-exchange materials. It is often the presence of LRW ligands significantly reduces the efficiency of sorption used sorbents with respect to, or other radionuclides. In nature, there are various minerals, committees consisting of both cation and anionobmennye groups, able to simultaneously act as a cation-and anion-exchange materials. These materials are layered double hydroxides (LDH). LDH are analogues of the natural mineral hydrotalcite.
Deposits of hydrotalcite is in Russia, Canada, USA, Norway, Sweden, Australia, France, Germany, the Czech Republic, etc.: Kovdor mine of alkaline-ultramafic rocks and carbonatites (Kola Peninsula), Hibiny (Kola Peninsula), Langban (Sweden ), Palabora (South Africa), etc.
Layered double hydroxides (LDH), representing a compound of: [M2+1-x M3+x (OH)2 [(Anionn-) x / n ּ mH2O], where M2 + and M3 + - cations in oxidation states 2+ and 3+ respectively, and Anionn-- virtually any anion or anionic complex . As analogues of natural mineral hydrotalcite and having layered structure with positively charged hydroxide layers [M2+1-x M3+x (OH) 2] x + and anions located in the interlayer space. To date, LDH obtained with different two-and trivalent metals, as well as various anions in their composition. For LDH with different anions in the interlayer space using following methods: 1) coprecipitation in the presence of a given anion, so as to obtain LDH containing only one type of anions in the interlayer space, 2) direct anion exchange, and 3) the interaction of a solution containing a specific Anionn-, with solid phase LMS 4) "melt" method.
The unit cell is formed hydrotalcite brusit-lake layers Mg (OH)2. Cations of magnesium in the brucite layer is surrounded by six oxygen atoms and the interlayer interaction is due to hydrogen bonds. With regard to double hydroxides, the substitution of divalent cations cations other Charged leads to the layers of positive charge, which is compensated by anions located in the interlayer space.
The structure of LDH is generally stable due to electrostatic interaction between positively charged hydroxide layers and interlayer anions, carrying negative charge. The vast majority of LDH crystallizes in the trigonal (rhombohedral) crystal system. One of the most important properties of LDH is the stability of their layered structure in a wide range of sizes of cations and anions. Samples of magnesium-aluminum LDH (LDH-Mg-Al), containing in the interlayer space of various anions with sizes ranging from 0.3 to 5 nm.
Another important feature is the thermal stability of LDH. Thermal decomposition of LDH occurs with preservation of their layered structure. This allows chemical reactions with their participation at elevated temperatures with virtually no destruction of the matrix, limiting the reaction zone.
In this paper, we synthesized compounds of hydrotalcite series. Carbonate form of LDH-Mg-Al-CO3 (hydrotalcite) was synthesized by precipitation from a solution of magnesium nitrate and aluminum (Mg: Al = 3: 1, total concentration of cations was 1 M) solution of carbonate and sodium hydroxide ([CO32-]: [OH -] = 1: 6, the total concentration of anions - 3 M) on the reaction:
3Mg2+ + Al3+ + 6OH- + ½(CO3)2- + mH2O→Mg3Al(OH)3(CO3)1/2.mH2O
In carrying out the synthesis of LDH-Mg-Al-CO3 poured into the reactor with stirring solutions of nitrates of aluminum and magnesium and precipitant solution dripped at the same time. The precipitate was kept in the mother liquor in 80ºC for 72 h. Then the precipitate was separated by centrifugation, dried in air at a temperature of 100-120ºC, repeatedly washed with water and again dried.
Synthesis of LDH-Mg-Nd-CO3, as well as mixed LDH-Mg-Al-Nd-CO3 with a different ratio of Al3 +: Nd3 + was carried out by the same method. In addition to the carbonate form of LDH-Mg-Al-CO3 LDH were synthesized samples-Mg-Al, containing in its composition NO3- and H2EDTA2- ions.
For the synthesized compounds were obtained by powder X-ray diffractometer ADP-10 from Philips with radiation СиКα, also were carried out thermogravimetric analysis, using derivatograph Q-1500 "MOM" (Budapest, Hungary). Heating the samples was carried out in Pt crucibles in air atmosphere at a speed 10 ºC /min. (fig. 1).
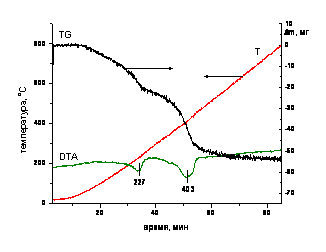
Fig. 1. The data of thermogravimetric analysis of LDH-Mg-Al-CO3
(sample weight - 119.7 mg)
With the ability to adapt different cations in brusit-lake layers, the possibility of intercalation of several types of anions in the interlayer space, layered double hydroxides can be used for catalysis, ion exchange, pharmacy and photochemistry. Particular interest is the use of LDH as geochemical barriers for radionuclides localization.
References:
Tretyakov, Yu.D., Lukashin, A.V., Eliseev, A.A. // Uspekhi Khimii. 2004. T. 73, № 9. pp. 974 - 998.
Kulyukhin, S.A., Krasavina, E.P., Rumer, I.A. et al // Radiochemistry. 2007. V. 49, № 5. pp. 437 - 441.